Write the Lewis structure for each compound, with the skeleton structure shown below. (a) (c)
Question:
Write the Lewis structure for each compound, with the skeleton structure shown below.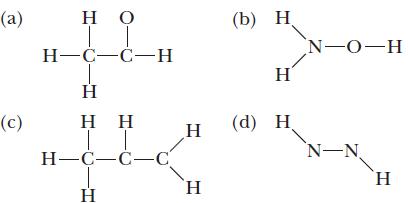
Transcribed Image Text:
(a) (c) Η Ο Ο H-C-C-Η Η Η Η T H=C=C=C Η (b) Η H Η H (d) Η N-0-H N-N H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
a b...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write the Lewis structure for each compound, with the skeleton structure shown below. (a) F-0-0-F (b) (c) C-C Cl Cl (d) H-C-0-H H-C-N-C-H
-
Write the Lewis structure for each compound, with the skeleton structure shown below. (a) H-C-C-H (c) H H H C-C-C-N (b) H-O-CI (d) H-0-0-H
-
Write the Lewis structure for each species, with the skeleton structure shown below. (a) (c) F F F F-B-F F c-c F F (b) (d) H H H H C-C-C-H H 1 C-C-C-C-H
-
Joshua Tree Inc. reported that at the end of 2012 net accounts receivable of 2,050 (the net value at the end of 2011 was 1,090). Their allowance for doubtful accounts at the end of 2012 was 150 (the...
-
(a) Derive linear density expressions for BCC [110] and [111] directions in terms of the atomic radius R. (b) Compute and compare linear density values for these same two directions for tungsten.
-
In Figure, one current is 8 A into the paper, the other current is 8 A out of the paper, and each curve is a circular path. ( a ) Find ? C B?d? for each path indicated. ( b ) Which path, if any, can...
-
Let FT,S t and T,S t denote the forward price and futures price at time t, respectively, for delivery at time T > t of a zero-coupon bond maturing at time S > T. Under the assumptions of the Vasicek...
-
The worker is using the bar to pull two pipes together in order to complete the connection. If he applies a horizontal force F to the handle of the lever, determine the moment of this force about the...
-
Exercise 6-15 Operating Leverage [LO6-1, LO6-8] Magic Realm, Inc., has developed a new fantasy board game. The coinpany sold 28,800 games last year at a selling price of $70 per game. Fixed expenses...
-
Draw a Lewis structure for each of the following molecules or ions. (a) CS 2 (b) BF 4 (c) HNO 2 (where the bonding is in the order HONO) (d) OSCl 2 (where S is the central atom)
-
Which atom in each of the following pairs has the greater electronegativity? Use only a periodic table to determine your answer. (a) Br, I (b) S, Cl (c) C, N
-
Balance each of the following unbalanced equations; then calculate the standard potential, E, and decide whether each is product-favored at equilibrium as written. (All reactions are carried out in...
-
4. Consider the RC circuit shown below: R C C2 The capacitors are connected in parallel. Use C = 120 F, C = 30F, R = 5002, and = 40V. The capacitors are initially uncharged and at t=0 the switch is...
-
The DuPont equation shows the relationships among asset management, debt management, and profitability of improving the firm's performance. Its equation is: ratios. Management can use the DuPont...
-
38. A pendulum bob of mass 0.200 kg is pulled to one side such that it has a height of 0.50 m relative to its rest position (that is, its lowest point). It is then released so that it swings back and...
-
Let A in K ^ ( n \ times n ) be an ( n \ times n ) - matrix. Show that there ist a basis B of K ^ n that consists of eigenvectors of A .
-
Using the "you" view is an effective way for writers to avoid taking on blame in business messages. O True False
-
Psilocybin is a hallucinogenic compound found in some mushrooms. Present a straight forward pathway for its biosynthesis from one of the aromatic amino acids. CH2 CH2 N(CH3)2 Psilocybin
-
An annual report of The Campbell Soup Company reported on its income statement $2.4 million as equity in earnings of affiliates. Journalize the entry that Campbell would have made to record this...
-
Determine the resultant internal loadings acting on the cross section at point E. The load D has a mass of 300 kg and is being hoisted by the motor M with constant velocity. -2 m 2 m m 0.1 m 0.1 m A...
-
Determine the resultant internal loadings acting on the cross section at point C in the beam. The load D has a mass of 300 kg and is being hoisted by the motor M with constant velocity. 2 m- E2 m- -2...
-
The hand crank that is used in a press has the dimensions shown. Determine the resultant internal loadings acting on the cross section at point A if a vertical force of 50 lb is applied to the handle...
-
ABC Insurance Company reported the following information on its accounting statements last year: What was ABC 's expense ratio last year
-
Calculate the current ratio and the quick ratio for the following partial financial statement for Tootsie Roll Note: Round your answers to the nearest hundredth
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Golden Corporation's current year income statement, comparative balance sheets, and...

Study smarter with the SolutionInn App


