Question: (a) With reference to Figure 6.19, what is the relationship between the number of nodes in an s orbital and the value of the principal
(a) With reference to Figure 6.19, what is the relationship between the number of nodes in an s orbital and the value of the principal quantum number?
Figure 6.19
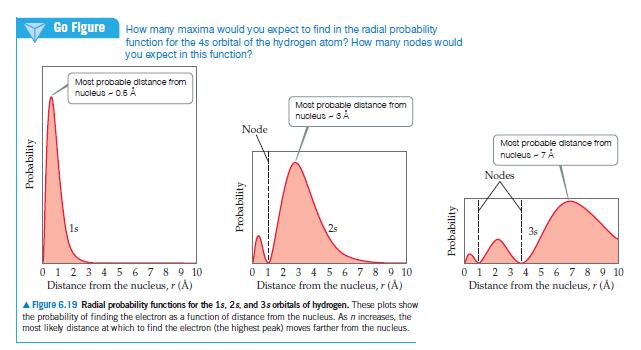
(b) Identify the number of nodes; that is, identify places where the electron density is zero, in the 2px orbital; in the 3s orbital.
(c) What information is obtained from the radial probability functions in Figure 6.19?
(d) For the hydrogen atom, list the following orbitals in order of increasing energy: 3s, 2s, 2p, 5s, 4d.
Probability Go Figure How many maxima would you expect to find in the radial probability function for the 4s orbital of the hydrogen atom? How many nodes would you expect in this function? Most probable distance from nucleus -0.5 A 1s 0 1 2 3 4 5 6 7 8 9 10 Distance from the nucleus, r () Node Probability Most probable distance from nucleus - SA 2s 0 1 2 3 4 5 6 7 8 9 10 Distance from the nucleus, r (A) A Figure 6.19 Radial probability functions for the 1s, 2s, and 3s orbitals of hydrogen. These plots show the probability of finding the electron as a function of distance from the nucleus. As n increases, the most likely distance at which to find the electron (the highest peak) moves farther from the nucleus. Probability Most probable distance from nucleus - 7 A Nodes 0 1 2 3 4 5 6 7 8 9 10 Distance from the nucleus, r ()
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
a With reference to Figure 619 what is the relationship between the number of nodes in an s orbital ... View full answer

Get step-by-step solutions from verified subject matter experts


