The radial probability function for a 2s orbital is shown here. Classify the following statements as either
Question:
The radial probability function for a 2s orbital is shown here.
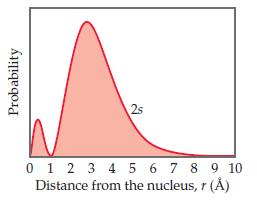
Classify the following statements as either true or false:
(a) There are two maxima in this function because one electron spends most of its time at an approximate distance of 0.5 Å from the nucleus and the other electron spends most of its time at an approximate distance of 3 Å from the nucleus.
(b) The radial probability function shown here and the probability density [Ψ(r)]2 both go to zero at the same distance from the nucleus, approximately 1 Å.
(c) For an s orbital, the number of radial nodes is equal to the principal quantum number, n.
Step by Step Answer:

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus





