Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C. (a)
Question:
Consider the three electronic transitions in a hydrogen atom shown here, labeled A, B, and C.
(a) Three electromagnetic waves, all drawn on the same scale, are also shown. Each corresponds to one of the transitions. Which electromagnetic wave (i), (ii), or (iii), is associated with electronic transition C?
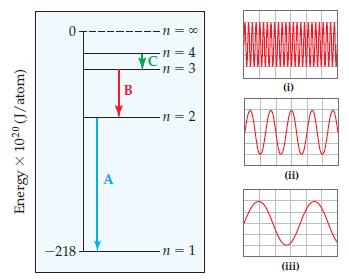
(b) Calculate the energy of the photon emitted for each transition.
(c) Calculate the wavelength of the photon emitted for each transition. Do any of these transitions lead to the emission of visible light? If so which one(s)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





