The molecule shown below is called furan. It is represented in typical shorthand way for organic molecules,
Question:
The molecule shown below is called furan. It is represented in typical shorthand way for organic molecules, with hydrogen atoms not shown.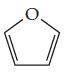
(a) What is the molecular formula for furan?
(b) How many valence electrons are there in the molecule?
(c) What is the hybridization at each of the carbon atoms?
(d) How many electrons are in the p system of the molecule?
(e) The C—C—C bond angles in furan are much smaller than those in benzene. The likely reason is which of the following:
(i) The hybridization of the carbon atoms in furan is different from that in benzene,
(ii) Furan does not have another resonance structure equivalent to the one above, or
(iii) The atoms in a five-membered ring are forced to adopt smaller angles than in a six-membered ring.
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





