The electric and magnetic forces on an electron in the CRT in Figure 30.7 are supposed to
Question:
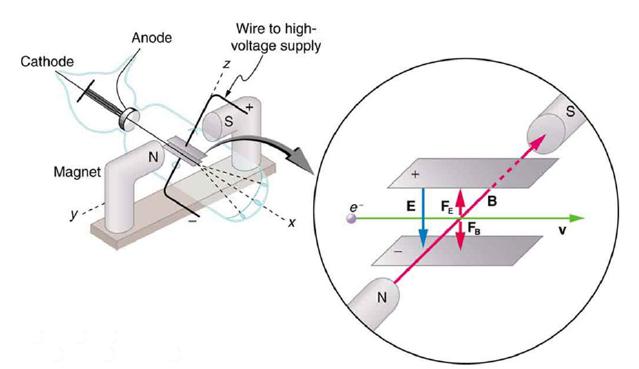
The electric and magnetic forces on an electron in the CRT in Figure 30.7 are supposed to be in opposite directions. Verify this by determining the direction of each force for the situation shown. Explain how you obtain the directions (that is, identify the rules used).
Data from figure 30.7
Transcribed Image Text:
Cathode Magnet Anode N Wire to high- voltage supply 2112 Z S N E FET FB B S
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The electric force on ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
In Figure 23.5, we analyzed the force exerted by the electric and magnetic fields of an electromagnetic wave on an electric charge at the surface of a material when the electric field has the...
-
Compare the magnitudes of the electric and magnetic forces on an electron that has attained a velocity of 10 7 m/s. Assume an electric field intensity of 10 5 V/m, and a magnetic flux density...
-
Dickens, Kristen, is enrolled as a doctoral student in the Counselor Education at the University of New Orleans. She is a registered counselor intern in the state of Louisiana and works at a...
-
Molecular weight data for some polymer are tabulated here. Compute the following: (a) The number-average molecular weight (b) The weight-average molecular weight. (c) If it is known that this...
-
How is the average number of days to collect accounts receivable computed? What information does the ratio provide?
-
You are provided with the following information about Bear River Inc.'s inventory for the month Instructions (a) Calculate the ending inventory and cost of goods sold using FIFO in (1) A perpetual...
-
Describe why stress occurs according to the demand-control model and the effort-reward imbalance model of stress. LO5
-
Standard Company has a relatively high profit margin on its sales, and Jewel Company has a substantially lower profit margin. Standard holds 55 percent of Jewels common stock and includes Jewel in...
-
Calculate Tax laibility NOI in year of sale Projected NOI one year after sale \ table [ [ 1 0 0 0 0 0 , 2 0 0 0 0 0 , 5 0 0 0 0 0 ] , [ 1 0 3 0 0 0 , 2 0 6 0 0 0 , 5 1 5 0 0 0 ] ] Acquisition price \...
-
(a) What is the distance between the slits of a diffraction grating that produces a first-order maximum for the first Balmer line at an angle of 20.0? (b) At what angle will the fourth line of the...
-
(a) Using the Pauli exclusion principle and the rules relating the allowed values of the quantum numbers (n, l, m l , m s ), prove that the maximum number of electrons in a subshell is 2n 2 . (b) In...
-
We investigate how the shape of the limacon curve r = b + cos depends on the constant b (see Figure 25). (a) Argue as in Exercise 63 to show that the constants b and b yield the same curve. (b) Plot...
-
How do you demonstrate resilience as a leader during times of crisis or uncertainty, and what steps do you take to bolster your team's resilience ?
-
What would you do if it becomes clear to you that the potential successor you were grooming is not going to make the grade as a supervisor? What are your next steps? Do you think this grooming is...
-
How do services and products differ? What kind of decisions do companies make regarding products and services? Why are brands important to marketers? How do marketing strategies change during the...
-
What leadership principles do you feel you possess that are important for APRNs to exhibit? What principles do you need to explore to be more confident in performing? Which leadership style do you...
-
Question 1- Where do you go in the Courier to find out your amount of leftover inventory for a specific product last round? Based on the production tab of the worksheet I gave you; how do you use...
-
A woman on a dock is pulling in a rope fastened to the bow of a small boast. If the women's hands are 10 feet higher than the point where the rope is attached to the boat and if she is retrieving the...
-
For what reason might an exporter use standard international trade documentation (letter of credit, draft, order bill of lading) on an intrafirm export to its parent or sister subsidiary?
-
A typical nuclear reactor generates 1000 MW (1000 MJ/s) of electrical energy. In doing so, it produces 2000 MW of waste heat that must be removed from the reactor to keep it from melting down. Many...
-
Which has the larger kinetic energy, a 10 g bullet fired at 500 m/s or a 75 kg student running at 5.5 m/s?
-
At what speed does a 1000 kg compact car have the same kinetic energy as a 20,000 kg truck going 25 km/h?
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


