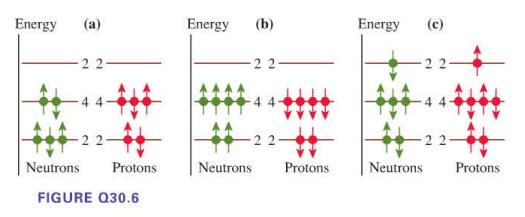
For each nuclear energy-level diagram in Figure Q30.6, state whether it represents a nuclear ground state, an
Question:
For each nuclear energy-level diagram in Figure Q30.6, state whether it represents a nuclear ground state, an excited nuclear state, or an impossible nucleus.
Transcribed Image Text:
Energy (a) 22 + 22- Neutrons Protons FIGURE Q30.6 Energy (b) -22 Neutrons Protons Energy Neutrons (c) 44- 22- Protons
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a This diagram represents an excited nuclear state The ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

College Physics A Strategic Approach
ISBN: 9780321595492
2nd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field
Question Posted:
Students also viewed these Physics questions
-
Seven possible transitions are identified on the energy level diagram in Figure Q29.20. For each, is this an allowed transition? If allowed, is it an emission or an absorption transition, and is the...
-
The CN molecule has been found in interstellar space. Assuming the electronic structure of the molecule can be described using the molecular orbital energy level diagram in Figure 9.16, answer the...
-
In a nuclear physics experiment, a proton is fired toward a Z = 13 nucleus with the diameter and neutron energy levels shown in Figure 40.17 . The nucleus, which was initially in its ground state,...
-
Differentiate between formal and behavioral roles, and describe how behavioral roles emerge during group interaction.
-
Merchandise is sold on account to a customer for $18,000, terms FOB shipping point, 3/10, n/30. The seller paid the transportation costs of $375. Determine the following: (a) Amount of the sale, (b)...
-
What would happen if, instead of adding value to the house, Tye attached a livestock barn, intended to house pigs, directly to the house and actually caused the value to deteriorate because of the...
-
If a company continues in existence because of the negligence of the auditors then it is right that those auditors should be responsible for its future losses as they would not have been incurred but...
-
1. Are the operations of Copper Kettle Catering conductive to the application of lean concepts and practices? Explain. 2. What, if any are the major barriers to implanting a lean system at Copper...
-
Cash Receipts Transactions Zebra Imaginarium, a retail business, had the following cash receipts during December 20--. The sales tax is 6%. Dec. 1 Received payment on account from Michael Anderson,...
-
A sample contains a mix of isotopes of an element. Using a spectrometer to measure the spectrum of emitted light will not reveal the mix of isotopes; analyzing the sample with a mass spectrometer...
-
A radioactive sample has a half-life of 10 s. 10,000 nuclei are present at t = 20 s. a. How many nuclei were there at t = 0 s? b. How many nuclei will there be at t = 40 s?
-
Under perfect competition _________profits are always zero in the long run. a) accounting b) economic c) both economic and accounting d) neither accounting or economic.
-
Are some values in the class data grossly different from all the others? If so, check for errors in calculation or procedure that would allow to objectively eliminate the data. 2. Do the range values...
-
An aging analysis of Uli Limited's accounts receivable at December 3 1 , 2 0 2 4 and 2 0 2 3 , showed the following: Number of Days Outstanding Accounts Receivable Estimated Percentage Uncollectible...
-
(Linear momentum) Two jets of liquid, one with specific gravity 1.00 and the other with specific gravity 1.33, collide and form one homogeneous jet as shown in the figure below. Determine (a) the...
-
1. Define a person-centered model of care in LTC facilities. 2. Describe two leadership behaviors and two leadership qualities most conducive to moving long-term care organizations toward more...
-
question 5 all parts 8+0.5 = 4. Consider a system with a lead compensator Ge(s) = +0.13 followed by a plant G(s) = 10 Determine a value for a gain K on the error signal such that the phase margin...
-
We will investigate the initial-value problem y' = y2, y(0) = 1. (a) Show that Picard's conditions hold. How large a region R can be found? (b) Draw the direction field and solution. (c) Solve the...
-
2. In the circuit given in Figure 2, i,(t) = 5.67cos(5t)A and v (t) = 70.71 cos(5t 60) V a) Find the equivalent load impedance. State whether the load is inductive or capacitive. b) Calculate the...
-
A wave travels with speed 200 m/s. Its wave number is 1.5 rad/m. What are its (a) Wavelength (b) Frequency?
-
You are standing at x = 0 m, listening to a sound that is emitted at frequency f 0 . The graph of FIGURE Q16.12 shows the frequency you hear during a 4-second interval. Which of the following...
-
The displacement of a wave traveling in the negative y-direction is D(y, t) = (5.2 cm) sin(5.5y + 72t), where y is in m and t is in s. What are the (a) Frequency, (b) Wavelength, (c) Speed of this...
-
WHAT IS PARTNERSHIP? CHARACTERISTICS ADMISSION, RETIREMENT , DEATH OF A PARTNER, DISSOLUTION OF PARTNERSHIP ADVANTAGES DISADVANTAGES
-
1 According to the author of the article, "Why are Increasing Numbers of Women Choosing to be Single?" the term spinsterhood was historically associated with : O Lower-status and less freedom O Legal...
-
An ex-husband can continue to claim a dependency, if qualified, for a father-in-law after a divorce. Choose one answer. a. True b. False

Study smarter with the SolutionInn App


