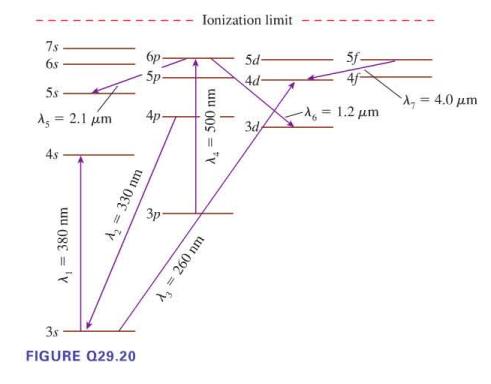
Seven possible transitions are identified on the energy level diagram in Figure Q29.20. For each, is this
Question:
Seven possible transitions are identified on the energy level diagram in Figure Q29.20. For each, is this an allowed transition? If allowed, is it an emission or an absorption transition, and is the photon infrared, visible, or ultraviolet? If not allowed, why not?
Transcribed Image Text:
7s 68 5s A = 2.1 μm 4s A₁ = 380 nm A₂ = 330 nm 3.s FIGURE Q29.20 бр 5p- 3p Ionization limit A₂ = 500 nm A₂ = 260 nm 5d- 4d- 3d/ 5f- -λ = 1.2 μm = 4.0 μm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Here is an analysis of the seven possible transitions identified on the energy level diagram in Figure Q2920 Transition Allowed Type of Transition Typ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

College Physics A Strategic Approach
ISBN: 9780321595492
2nd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field
Question Posted:
Students also viewed these Physics questions
-
Figure 28.19 shows part of the energy level diagram for the electrons in an imaginary atom. The arrows represent three transitions between the energy levels. For each of these transitions: a....
-
Question 1 [34 marks] Read the scenarios below for the year ending 28 February 2023 and answer the following question. A. The following is the income statement of Axe Ltd for the financial year...
-
In a set of experiments on a hypothetical one-electron atom, you measure the wavelengths of the photons emitted from transitions ending in the ground state (n = I), as shown in the energy level...
-
Were Napoleon's territorial ambitions significantly different from pre-1799 conquests? If so, where?What were Napoleon's most significant domestic accomplishments in France? Consider the interesting...
-
Zippy Computers announced strong fourth quarter results. Sales and earnings were both above analysts expectations. You notice in the newspaper that Zippys stock price went up sharply on the day of...
-
The dean of students wants to see whether there is a significant difference in ages of resident students and commuting students. She selects a sample of 50 students from each group. The ages are...
-
Distributed processing also occurs for other cognitive functions, such as memory, decision making, and problem solving. A basic principle of cognition is that different cognitive functions often...
-
Stockholders equity accounts and other related accounts of Gonzales Company as of January 1, 20--, the beginning of its fiscal year, are shown below. Preferred stock subscriptions...
-
ABC Corporation is a new company that buys and sells office supplies. Business began on January 1, 2016. Given on the first two tabs are ABC's 12/31/16 Unadjusted Trial Balance and a list of needed...
-
List the quantum numbers of (a) All possible 3p states (b) All possible 3d states.
-
Show, by actual calculation, that the Bohr radius is 0.0529 nm and that the ground-state energy of hydrogen is -13.60 eV.
-
List the combinations of yogurt and berries that Jerry can afford. Draw a graph of his budget line with the quantity of berries plotted on the x axis. Jerry has $12 a week to spend on yogurt and...
-
Share your thoughts on the descriptions of coaching versus mentoring. Discuss which technique you personally find more helpful, incorporating your peers' example scenarios if possible. Provide...
-
Hanung Corp has two service departments, Maintenance and Personnel. Maintenance Department costs of $380,000 are allocated on the basis of budgeted maintenance-hours. Personnel Department costs of...
-
Discuss difference between nominal interest rate and real interest rate. Explain why real interest rate is more important than the nominal interest rate using your answer to Question 1 of the...
-
Refer to Figure 14-1. How would an increase in the money supply move the economy in the short and long run?
-
1) Special Relativity. Statement: Imagine this situation: Alice stands in New York City while Bob, aboard a plane departing from Boston, directly crosses over Alice at t=0. Disregard the vertical...
-
Let H be an n n nonsingular matrix. What basis of Rn does H change to the standard basis?
-
Open Text Corporation provides a suite of business information software products. Exhibit 10-9 contains Note 10 from the companys 2013 annual report detailing long-term debt. Required: a. Open Text...
-
A rocket in deep space has an exhaust-gas speed of 2000 m/s. When the rocket is fully loaded, the mass of the fuel is five times the mass of the empty rocket. What is the rockets speed when half the...
-
A tennis player swings her 1000 g racket with a speed of 10 m/s. She hits a 60 g tennis ball that was approaching her at a speed of 20 m/s. The ball rebounds at 40 m/s. a. How fast is her racket...
-
A 60 g tennis ball with an initial speed of 32 m/s hits a wall and rebounds with the same speed. FIGURE P11.39 shows the force of the wall on the ball during the collision. What is the value of F max...
-
Lenitnes Company is considering an investment in technology to improve its operations. The investment will require an initial outlay of $267,000 and will yield the following expected cash flows....
-
E 5-9 Present value; annuities L05-8 Using the appropriate present value table and assuming a 12% annual interest rate, determine the present value on December 31, 2021, of a five-period annual...
-
QUESTION 16 One of your tax clients has asked you a question about terminating his partnership interest. All of the following may result in the termination of a partnership interest, except :...

Study smarter with the SolutionInn App


