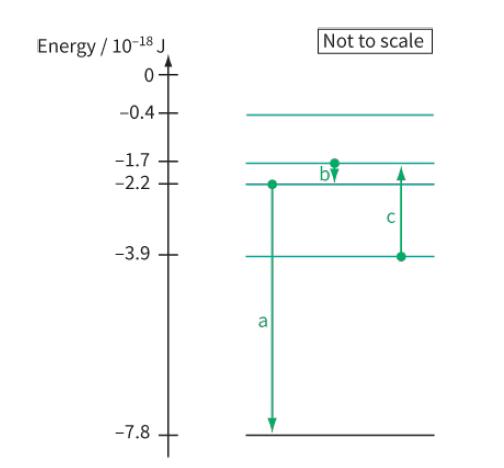
Figure 28.19 shows part of the energy level diagram for the electrons in an imaginary atom. The
Question:
Figure 28.19 shows part of the energy level diagram for the electrons in an imaginary atom. The arrows represent three transitions between the energy levels. For each of these transitions:
a. Calculate the energy of the photon
b. Calculate the frequency and wavelength of the electromagnetic radiation (emitted or absorbed)
c. State whether the transition contributes to an emission line in the spectrum or an absorption line in the spectrum.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:





