Question: For the system shown in Figure 28.19, which are possible energies of emitted photons? There may be more than one correct answer. A. 40eV B.
For the system shown in Figure 28.19, which are possible energies of emitted photons? There may be more than one correct answer.
A. 40eV
B. 50eV
C. 60 eV
D. 70eV
E. 80eV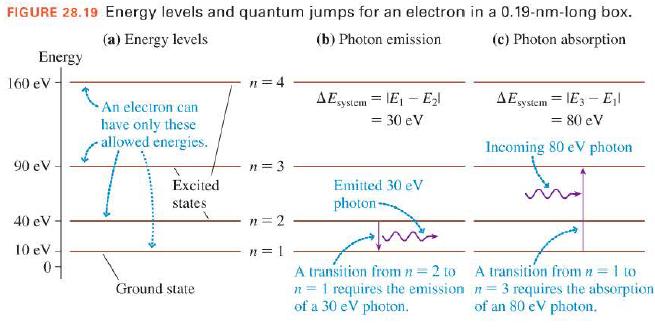
FIGURE 28.19 Energy levels and quantum jumps for an electron in a 0.19-nm-long box. (a) Energy levels (b) Photon emission (c) Photon absorption Energy 160 ev n=4 = An electron can AE system E- El have only these = 30 eV AE system E3-El = 80 eV - allowed energies. Incoming 80 eV photon 90 eV 11=3 Excited states. Emitted 30 eV photon- 40 eV - n=2 10 eV n = 1 0- Ground state A transition from n = 2 to 12= 1 requires the emission of a 30 eV photon. A transition from n = 1 to n 3 requires the absorption of an 80 eV photon.
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
B D and E The emitted photons energies must be the di... View full answer

Get step-by-step solutions from verified subject matter experts


