Ordinary soil contains typically 1 part per million (ppm) of uranium by mass. (a) How many uranium
Question:
Ordinary soil contains typically 1 part per million (ppm) of uranium by mass.
(a) How many uranium nuclei are in the top 10 m of soil under a typical house (20 m 20 m)?
(b) Only 0.72% of the nuclei in part (a) are 23592U; the rest are 23892U, which has a much longer half-life. How many 23 92 5U nuclei are in the soil under this house? How many 23892U nuclei are in the soil under this house?
(c) How many of the 23892U nuclei in part (b) undergo radioactive decay over a period of one day?
(d) How many of the 23886U nuclei in part (b) undergo radioactive decay over a period of one day?
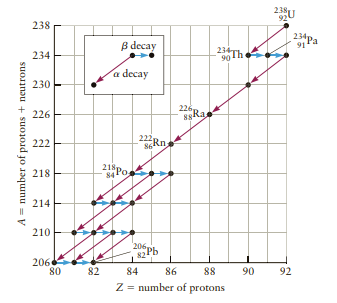
(e) The decay of 238 92U leads to radon gas (22286Rn; see Fig. 30.9). If all the 23892U decays to make 22286Rn, how many 22286Rn nuclei are produced under the house in one month?
(f) If all the 22286Rn nuclei in part (e) seep into the house above, what is the activity in the house?
Figure 30.9
Step by Step Answer:

College Physics Reasoning and Relationships
ISBN: 978-0840058195
2nd edition
Authors: Nicholas Giordano





