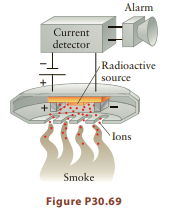
Some smoke detectors (Fig. P30.69) use the radiation produced by the decay of 24 95 1Am (half-life
Question:
Some smoke detectors (Fig. P30.69) use the radiation produced by the decay of 24 95 1Am (half-life 432 yr) to ionize air molecules, which in turn produces a steady current across two electrodes. If smoke enters the region of ionization, the alpha particles are absorbed by the smoke particulates, reducing the current accross the electrodes. The electronic circuitry then sounds the alarm when it senses any current reduction. How long does it take for the activity of 24195Am to drop to 1% of its initial value? Why is it important to recycle smoke detectors rather than discard them?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics Reasoning and Relationships
ISBN: 978-0840058195
2nd edition
Authors: Nicholas Giordano
Question Posted:





