Find the mass of the 238 U + ions in terms of v, B, D, and universal
Question:
Find the mass of the 238U+ ions in terms of v, B, D, and universal constants.
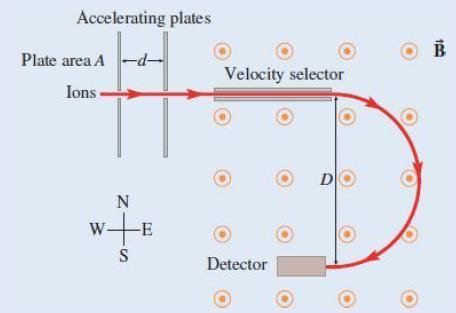
Transcribed Image Text:
Accelerating plates Plate area A -d- Ions N w+ -E S Velocity selector D Detector
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
a Here the potential energy of Will convert to kineti...View the full answer

Answered By

Suneel Kumar
I completed my masters in Accountancy and finance and started doing job . I have a good enough interest and expertise in teachin g students. Exploring subject through a tutor will make me a good knowledge.
0.00
0 Reviews
10+ Question Solved
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:
Students also viewed these Sciences questions
-
Find the mass of the surface z = 1 - (x2 + y2) / 2 over 0 ( x ( 1, 0 ( y ( 1, if ((x, y, z) = kxy?
-
Find the mass of 0C ice that 10 g of 100C steam will completely melt.
-
Find the mass of the following thin bars. A bar on the interval 0 x 9 with a density (in g/cm) given by (x) = 3 + 2x.
-
Consider an electric motor with a shaft power output of 20 kW and an efficiency of 88 percent. Determine the rate at which the motor dissipates heat to the room it is in when the motor operates at...
-
Many economists believe that China will soon achieve superpower status because of its economic reforms, along with the work ethic and high education of its population. How is the rise of China...
-
On July 1, Jones Corporation had the following capital structure: Common Stock, par $ 1; 8,000,000 authorized shares, 100,000 issued and outstanding.. $ 100,000 Additional Paid-in Capital 90,000...
-
Understand how fashion shows are a fundamental part of the industry and its buying cycle. LO.1
-
1. What was the total purchase price/ enterprise value of the transaction? 2. Why did Exxon Mobils shares decline and XTO Energys shares rise substantially immediately following the announcement of...
-
please help fast! print budgetJexpected to use 2700 ft and for it cost $2 per de cordones wide one does the company pod towy 980d amount of greet materials. This month pureed 2.000 of material. The...
-
Refer to the data in Exercise 61 for Ida Company. The absorption costing income statement prepared by the companys accountant for last year appears as shown: Sales . . . . . . . . . . . . . . . . . ....
-
(a) How much energy is stored in the inductor at t = 0? (b) What is the instantaneous rate of change of the inductors energy at t = 0? (c) What is the average rate of change of the inductors energy...
-
Two parallel wires in a horizontal plane carry currents I 1 and I 2 to the right. The wires each have length L and are separated by a distance d. Find the magnitudes and directions of the (a) The...
-
(a) In conventional mold design, straight cooling (heating) passages are bored through the mold in a location where the passages will not interfere with the molded part. Determine the initial heating...
-
What are factors which hamper the promotion of an entrepreneurial culture in South Africa?
-
Please Help P/R End Date 2/8/2019 Company Name: Prevosti Farms and Sugarhouse Check Date 2/13/2019 Tax Name M/S # of W/H Hourly Rate or Period # of Regular # of Overtime # of Holiday Wage Hours Hours...
-
Read the description of following adjustments that are required at the end of the accounting period for Hubbard Repair Services, a new firm. Determine the account and amount to be debited and the...
-
Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that time has just flown by; over twenty-four years...
-
Here are summary statistics for randomly selected weights of newborn girls: n = 36, x = 3180.6 g, s = 700.5 g. Use a confidence level of 99% to complete parts (a) through (d) below. a. Identify the...
-
Compare and contrast consultative, democratic, and free-rein styles of participative leadership.
-
A regular deposit of $100 is made at the beginning of each year for 20 years. Simple interest is calculated at i% per year for the 20 years. At the end of the 20-year period, the total interest in...
-
How would you use 1 H NMR spectroscopy to distinguish between the following compounds? (a) (b) (c) (d) (e) (f) . . . CI
-
A chemist was attempting to achieve the following transformation: Mass spectrometry was then used to verify that the molecular formula of the major product was C 7 H 15 Br, just as expected. However,...
-
Propose a structure that is consistent with each of the following 1 H NMR spectra. In each case, the molecular formula is provided. (a) (b) (c) (d) (e) (f) Proton NMR C,H,,0 i ppm Integration Values...
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


