Question: Propose a structure that is consistent with each of the following 1 H NMR spectra. In each case, the molecular formula is provided. (a) (b)
Propose a structure that is consistent with each of the following1H NMR spectra. In each case, the molecular formula is provided.
(a)
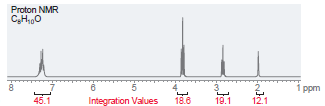
(b)
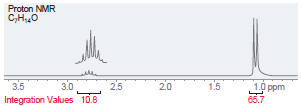
(c)
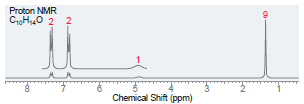
(d)
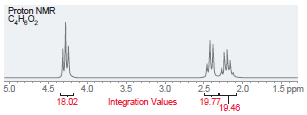
(e)
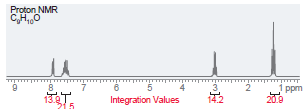
(f)
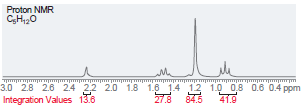
Proton NMR C,H,,0 i ppm Integration Values 18.8 12.1 19.1 45.1 Foo Proton NMR C,H0 3.5 Integration Values 10.8 1.0 ppm 85.7 2.5 2.0 1.5 3.0
Step by Step Solution
3.26 Rating (161 Votes )
There are 3 Steps involved in it
a ... View full answer

Get step-by-step solutions from verified subject matter experts


