Radioactive decays into . Which of these particles is released in the decay? (a) A proton (b)
Question:
Radioactive 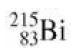 decays into
decays into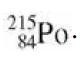 . Which of these particles is released in the decay?
. Which of these particles is released in the decay?
(a) A proton
(b) An electron
(c) A positron
(d) An alpha particle
(e) A neutron
(f) None of the above
Transcribed Image Text:
215, 83 Bj
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
c A positron Explanation The decay process shown in the images is called beta decay ...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:
Students also viewed these Sciences questions
-
10. Which of the following statement is correct? Ans : O Citing online source: Provide the author of the work, the title of the posting in quotation marks, the web site name in italics, the...
-
What is the body of the loop to have the following output : 268 20 11 56 ? * int [] a = { 1, 5, 7, 19, 10, 55 }; for (int i = 0; i < a.length ; i += 2) a[i]++; for (int i = 1; i < a.length ; i += 2)...
-
5. Hanin Inc. recently reported $350,000 of sales, $75,500 of operating 4 points costs other than depreciation, and $10,200 of depreciation. The company had $16,500 of outstanding bonds that carry a...
-
A clinical study is established to determine if the results of a screening stress test can be used as a predictor of the presence of heart disease. The study enrolls 100 participants who undergo a...
-
Some cruise ship passengers are given magnetic bracelets, which they agree to wear in an attempt to eliminate or diminish the effects of motion sickness. Others are given similar bracelets that have...
-
Consider the closed-loop system in Figure P5.21. Determine values of the parameters k and a so that the following specifications are satisfied: (a) The steady-state error to a unit step input is...
-
Milden Company is a merchandiser that plans to sell 12,000 units during the next quarter at a selling price of $100 per unit. The company also gathered the following cost estimates for the next...
-
Around June 2005, PPI purchased three pallets of computer wafers from Omneon Video Graphics. PPI requested that Omneon ship the wafers directly to the City University of New York, the end purchaser...
-
Given the information below, what is the gross profit? Sales revenue Accounts receivable Ending inventory Cost of goods sold Sales returns $315,000 57,000 114,000 239,000 30,000 Multiple Choice O...
-
Q1) For a certain diatomic molecule, two of the pure-rotational absorption lines are at 806.65 GHz and 921.84 GHz and there are no pure-rotational lines between these two lines. Find the initial /...
-
Which of these are appropriate units for the decay constant of a radioactive nuclide? (a) s (b) Ci (c) rad (d) s1 (e) rem (f) MeV
-
Rank these nuclides in decreasing order of the number of neutrons: (a) He (b) He (c) H (d) Li (e) B (f) Li
-
TheaterSeat Store chairs with or without subwoofer speakers. Identify each product by its level of customer involvement. A. Make-to-stock B. Assemble-to-order C. Make-to-order
-
Consider the following account balances (in thousands) for the Shaker Corporation In the Dec 31.2021 Cash $200,000 and Capital $2,000,000 and Retained earnings $1,500,000 The balances of raw...
-
Unless otherwise stated, assume gravitational acceleration g = 9.81 m/s and the density of water to be 1000 kg/m. Unless otherwise stated, give all numerical answers to 3 significant figures, such as...
-
The purpose of this installment is to classify stock, bond, and mutual fund investments, explore tools for their evaluation and select these securities based on your investment philosophy and goals....
-
Jackson County Senior Services is a nonprofit organization devoted to providing essential services to seniors who live in their own homes within the Jackson County area. Three services are provided...
-
Caldwell (2003) explores differences between the roles of leaders and managers. "Leaders...envision, initiate, or sponsor strategic change of a far-reaching or transformational nature. In contrast,...
-
The maximum numbers of passenger vehicle lanes at 16 Canadian border ports of entry (Source: U.S. Customs and Border Protection) 8 6 1 0 3 6 1 1 17 2 2 6 1 1 0 3 1 9 10 5 Find the mean, the median,...
-
The area of square PQRS is 100 ft2, and A, B, C, and D are the midpoints of the sides. Find the area of square ABCD. B A
-
Consider the following reaction: (a) Use Table 6.1 to estimate ÎH for this reaction. (b) ÎS of this reaction is positive. Explain. (c) Determine the sign of ÎG. (d) Is the sign of...
-
Consider the following four energy diagrams: (a) Which diagrams correspond with a two-step mechanism? (b) Which diagrams correspond with a one-step mechanism? (c) Compare energy diagrams A and C....
-
For each of the following reactions identify the arrow-pushing pattern that is being utilized: a. b. c. d. H,
-
18. Suppose that Maxima shares are selling for $10 per share and you own a call option to buy Maxima shares at $7.50. The intrinsic value of your option is:
-
ABC Insurance Company reported the following information on its accounting statements last year: What was ABC 's expense ratio last year
-
Calculate the current ratio and the quick ratio for the following partial financial statement for Tootsie Roll Note: Round your answers to the nearest hundredth

Study smarter with the SolutionInn App


