Rank these nuclides in decreasing order of the number of neutrons: (a) He (b) He (c) H
Question:
Rank these nuclides in decreasing order of the number of neutrons:
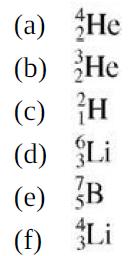
Transcribed Image Text:
(a) He (b) He (c) H (d) Li (e) B (f) Li
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:
Students also viewed these Sciences questions
-
Rank the following carbocations in decreasing order of stability. Classify each as primary, secondary, or tertiary. (a) The isopentyl cation, (b) The 3-methyl-2-butyl cation, (c) The 2-methyl-2-butyl...
-
Rank the following radicals in decreasing order of stability. Classify each as primary, secondary, or tertiary. (a) The isopentyl radical, (b) The 3-methyl-2-butyl radical, (c) The 2-methyl-2-butyl...
-
Rank the given solvents in decreasing order of their ability to dissolve each compound. (a) NaOAc (b) (c) naphthalene OH 2-naphthol
-
Saeed does not keep proper books of account for his business but he has provided the following details of his assets and liabilities. Further information 1. Land and buildings have been revalued at...
-
What is a placebo, and why is it important in an experiment to test the effectiveness of a drug?
-
Photovoltaic arrays (solar cells) generate a DC voltage that can be used to drive DC motors or that can be converted to AC power and added to the distribution network. It is desirable to maintain the...
-
M. K. Gallant is president of Kranbrack Corporation, a company whose stock is traded on a national exchange. In a meeting with investment analysts at the beginning of the year, Gallant had predicted...
-
Due to its experience rating, Ianelli, Inc., is required to pay unemployment taxes on its payroll as follows: 1. Under SUTA for Arizona on taxable payroll of $28,000, the contribution rate is 3.9%....
-
PREPARE THE ADJUSTING JOURNAL ENTRIES REQUIRED Because Natalie has had such a successful first few months, she is considering other opportunities to develop her business. One opportunity is to become...
-
Capital Edge Company has found that, historically, 0.5% of its current accounts receivable, 3% of accounts 1 to 30 days past due, 4.5% of accounts 31 to 60 days past due, 8% of accounts 61 to 90 days...
-
Radioactive decays into . Which of these particles is released in the decay? (a) A proton (b) An electron (c) A positron (d) An alpha particle (e) A neutron (f) None of the above 215, 83 Bj
-
For all stable nuclei, (a) the mass of the nucleus is less than Zmp + (A - Z)mn- (b) the mass of the nucleus is greater than Zmp + (A - Z)mn. (c) the mass of the nucleus is equal to Zmp + (A - Z)mn-...
-
Do you think transaction processing systems differ significantly between service and manufacturing industries? Are they equally important to both sectors?
-
1. What gives stainless steels their good corrosion resistant properties? 2. Which stainless steel is the lowest cost and why? 3. What are some characteristics of Nickel Alloys? 4. What are the 2...
-
Problem 4. Determine the motion of a two-dimensional linear oscillator of potential energy V = kr
-
5 Informatics solutions in the "complex and catastrophic" end of the population-risk spectrum must support which type of services/functions? 1 point Intensive case management Wellness program
-
What are the characteristics of products that Otis Trains produces? What are order qualifiers and winners? Explain at least three advantages and three drawbacks of offshoring to JLPTC. What risks are...
-
Find the angle and length of the resulting vector for the given d and e vectors by the analytical method. After that, find the parameters of the resulting vector for the three vectors. In the answer,...
-
The 20162017 tuition and fees (in thousands of dollars) for the top 14 universities in the U.S. 45 47 52 49 55 48 48 51 51 50 51 48 51 51 Find the mean, the median, and the mode of the data, if...
-
SBS Company have received a contract to supply its product to a Health Care Service Hospital. The sales involve supplying 1,250 units every quarter, the sales price is RM 85 per unit. The Client...
-
Rank the three carbocations shown in terms of increasing stability: a. b.
-
For the following mechanism, identify the sequence of arrow-pushing patterns: CI CI CI CI Lci-Al-CI je CI
-
Draw two constitutional isomers that have molecular formula C 3 H 7 Cl.
-
Glencove Company makes one model of radar gun used by law enforcement officers. All direct materials are added at the beginning of the manufacturing process. Information for the month of September...
-
Larren Buffett is concerned after receiving her weekly paycheck. She believes that her deductions for Social Security, Medicare, and Federal Income Tax withholding (FIT) may be incorrect. Larren is...
-
The major justification for adding Step 0 to the U.S. GAAP impairment test for goodwill and indefinite lived intangibles is that it: A. Saves money spent estimating fair values B. Results in more...

Study smarter with the SolutionInn App



