Rank the six gases of Problem 43 in order of the total translational kinetic energy, greatest to
Question:
Rank the six gases of Problem 43 in order of the total translational kinetic energy, greatest to least.
Data From Problem 43
Six cylinders contain ideal gases (not necessarily the same gas) with the properties given (P = pressure, V = volume, N = number of molecules). Rank them in order of temperature, highest to lowest.
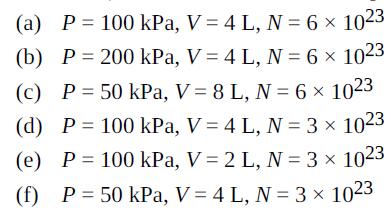
Transcribed Image Text:
(a) P = 100 kPa, V = 4 L, N = 6 x 1023 (b) P = 200 kPa, V = 4 L, N = 6 x 1023 (c) P= 50 kPa, V = 8 L, N = 6 x 1023 (d) P = 100 kPa, V = 4 L, N = 3 x 1023 (e) P = 100 kPa, V = 2 L, N = 3 x 1023 (f) P = 50 kPa, V = 4 L, N = 3 x 1023
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
Strategy Expression for ideal gas equation Here is the pressure of the gas is the volume of the gas is the number of molecules is the temperature of t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:
Students also viewed these Sciences questions
-
Rank the molecules below from lowest to highest according to their ability to diffuse across a lipid bilayer. Explain your rationale. H CCH CH2 NH H H3C NH
-
Rank the following six transactions from lowest to highest transaction costs. Explain your ranking by reference to the costs of search, bargaining, and enforcement. a. Getting married. b. Buying an...
-
Six cylinders contain ideal gases (not necessarily the same gas) with the properties given (P = pressure, V = volume, N = number of molecules). Rank them in order of temperature, highest to lowest....
-
1. Prepare program using threads in java that can print 10 times the numbers 1,2,3,4,5 in a series. 2. Prepare program using threads and a semaphore in java that can print the numbers 1,2,3,4,5 in a...
-
What total amount must be paid on July 4 to settle invoices dated June 20 for $485, June 24 for $367, and June 30 for $722, all with terms 11/2 /10, n/30?
-
What is 360-degree feedback? What advantages might it have over more traditional performance appraisal systems that use only downward feedback? What are some of the problems that could occur in using...
-
2. What are some advantages of the style(s) you use most often? Some disadvantages?
-
Refer to the financial statements of American Eagle Outfitters given in Appendix B at the end of this book. At the bottom of each statement, the company warns readers to "Refer to Notes to...
-
How does the equation for valuing a bond change if semiannual payments are made? Find the value of a 10-year, semiannual payment, 12% coupon bond if nominal rd = 11%.
-
Complete Keith's tax return including all required schedules and forms using prince edward island as province, using the fillable forms package. Taxpayers Information Taxpayer #1 Name: Keith Dox...
-
What is the total translational kinetic energy of the gas molecules of air at atmospheric pressure that occupies a volume of 1.00 L?
-
Consider the expansion of an ideal gas at constant pressure. The initial temperature is T0 and the initial volume is V 0 . (a) Show that V/V 0 = T, where = 1/T 0 . (b) Compare the coefficient of...
-
The subjects for the study described in Example 12 were evaluated for abstinence from cigarette smoking at the end of 12 months. The table shows the percentage in each group that were abstaining. a....
-
Coaching for Performance Develop a strategy for how you will approach the coaching session with the employee, including what you plan to discuss and any questions you may have when you debrief....
-
For the following exercises, find the derivatives of the given functions: 1. y=x-secx+1 2. y = 3 cscx+ 5 3. f(x) = x cotx 4. f(x) = secx I 5. y=
-
1. why does Amazon use ERP system? How does ERP system work for Amazon? what are the benefit and drawbacks of using ERP for Amazon? 2. what are 5 industry best practices across Finance,...
-
A rigid vessel contains afuel gasconsisting of a methane (CH4) and ethane (C2H6) mixture. The pressure in the vessel is found to be 0.30 bar.Air is added to the vessel until the total pressure...
-
Do you agree with this discussion post? My article discusses decision-making tools in Project Management (PM). "In research and development (R&D), project management (PM) decision-making tools are...
-
A company reports purchases of $388,000, a beginning accounts payable balance of $27,000, and an ending accounts payable balance of $48,000. All purchases were on account. The companys accounts...
-
4. Jobe dy -Y 2 et by
-
Carbon-14 dating is used to date a bone found at an archaeological excavation. If the ratio of C-14 to C-12 atoms is 3.25 1013, how old is the bone?
-
A sample of radioactive 21483Bi, which has a half-life of 19.9 min, has an activity of 0.058 Ci. What is its activity 1.0 h later?
-
The activity of a sample containing radioactive 108Ag is 6.4 104 Bq. Exactly 12 min later, the activity is 2.0 103 Bq. Calculate the half-life of 108Ag.
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...
-
Reporting Financial Statement Effects of Bond Transactions (please show me how you got the answers) Lundholm, Inc., which reports financial statements each December 31, is authorized to issue...

Study smarter with the SolutionInn App


