The diagram below shows a hot water storage tank. The water is heated by an electric immersion
Question:
The diagram below shows a hot water storage tank. The water is heated by an electric immersion heater at the bottom.
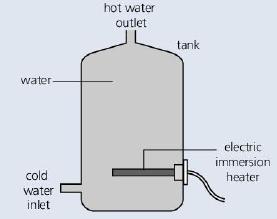
a. How could heat loss from the tank be reduced? What materials would be suitable for the job?
b. Why is the heater placed at the bottom of the tank rather than the top?
c. The heater has a power output of 3 kW.
i. What does the 'k’ stand for in 'kW'?
ii. How much energy (in joules) does the heater deliver in one second?
iii How much energy (in joules) does the heater deliver in 7 minutes?
d. The tank holds 1.00 kg of water The specific heat capacity of water is 4200 J/(kg °C).
i. How much energy (in joules) is needed to raise the temperature of 1 kg of water by 1 °C?
ii. How much energy (in joules) is needed to L raise the average temperature of all the water in the tank by 1 °C?
iii. If the heater is switched on for 7 minutes, what is the average rise in temperature of the water in the tank (assuming that no heat is lost)?
Step by Step Answer:






