For the emitter follower network of Fig. 4.135 a. Find I B , I C , and
Question:
For the emitter follower network of Fig. 4.135
a. Find IB, I C , and IE .
b. Determine VB , VC , and VE .
c. Calculate V BC and VCE.
Fig. 4.135
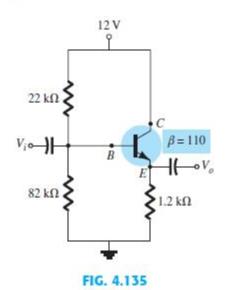
Transcribed Image Text:
22 ΚΩ . V₁o-H 82 ΚΩ , 12V FIG. 4.135 C β=110 Hov • 1.2 ΚΩ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
a Apply voltage divider formula at base terminal the voltage at base terminal Vb eq...View the full answer

Answered By

Beatrice Jeptoo
I worked as an Asst. Professor and now currently working as a Research Scholar in Indian Institute of Technology Tirupati. I am interested solve the related to digital domain, digital IC design, Verilog hdl, etc..
0.00
0 Reviews
10+ Question Solved
Related Book For 

Electronic Devices And Circuit Theory
ISBN: 9781292025636
11th Edition
Authors: Robert Boylestad, Louis Nashelsky
Question Posted:
Students also viewed these Engineering questions
-
Determine the VC and IB for the network of Fig. 4.135. 22 V 2.2 k 82 k = 220 16 k 0.75 k
-
Determine IE and VC for the network of Fig. 4.136. 8V 33 ko TE 3.9 k2
-
Determine the level of VE and IE for the network of Fig. 4.124. 330 k = 120 1.2 k
-
Given the following data: Calculate ÎH for the reaction On the basis of enthalpy change, is this a useful reaction for the synthesis of ammonia? AH - 92 kJ () + AH = -484 k (g) ON OH 88
-
Using sound bond valuation principles, explain in terms of present value why zero coupon bonds sell at such a deep discount to the par value.
-
The value of a forward contract that you have been holding for the last six months is $50 today. It matures in three more months. If todays spot price is $100 and the underlying interest rate is 5...
-
Assume a U.S. company makes a credit sale to a foreign customer that is required to make payment in its foreign currency. In the current period, the exchange rate is $1.40 on the date of the sale and...
-
Mountain Mash produces ice cream for wholesale distribution to grocers, restaurants, and independent ice cream shops. March, April, May, June, and July are busy months for the company as its...
-
QUESTION 19 JHS Inc. has reported the following data: Purchases of Raw Material $5,400 Freight In 150 Property Tax 950 Ending Inventory of Raw Materials 1,700 Beginning Inventory of Raw Materials...
-
Dewin Auer Best (DAB) CPAs has an audit and tax client named Meyers, Inc. Meyers is a closely held C corporation whose majority shareholder, Alicia Meyers, is also its CEO. Alicia also is a tax...
-
For the network of Fig. 4.136 , determine: a. I B . b. I C . c. V CE . d. V C . Fig. 4.136 9.1 9+16 V '12 le + = 80 15 6-12V FIG. 4.136
-
For the collector-feedback configuration of Fig. 4.129, determine: a. I B . b. I C . c. V C Fig. 4.129 270 W +16V 13.6 + Ic Vc = 120 1.2
-
Consider the situation given in Example 17.3. After the Series C investment by Owl, is the Series A implied LP valuation lower than its original LP cost?
-
Explain why its important to study management.
-
Wildhorse has not logged since 2016. If Wildhorse logged and sold 1,062,000 board feet of timber in 2027, when the timber cruise (appraiser) estimated 5,900,000 board feet, determine the cost of...
-
Y = AK[1-a R P = QAKa-1[1-a W P = (1 -Q) AKL-a 1= 14 1 -4 Y = C
-
Inferring Transactions from Financial Statements (FSET) Wired.com Inc. is a large e-commerce company, with over $31 billion in revenues for the fiscal year ended December 31, 20X2. For the year ended...
-
Finding Standard Deviation from a Frequency Distribution. In Exercises 37-40, refer to the frequency distribution in the given exercise and compute the standard deviation by using the formula below,...
-
Kim works for a clothing manufacturer as a dress designer. During 2018, she travels to New York City to attend five days of fashion shows and then spends three days sightseeing. Her expenses are as...
-
Coastal Refining Company operates a refinery with a distillation capacity of 12,000 barrels per day. As a new member of Coastal's management team, you have been given the task of developing a...
-
A 5.0-kg steel gear is heated to 150C and then placed into a 0.5-gal container of water at 10C. What is the final temperature of the metal and water?
-
Give two examples each of engineering applications where heat would be transferred primarily through conduction, convection, and radiation.
-
A hollow square box is made from 1-ft 2 sheets of a prototype insulating material that is 1 in. thick. Engineers are performing a test to measure the new materials thermal conductivity. A 100-W...
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


