(a) Verify Equation 36.8 by considering a single-electron atom with nuclear charge Ze instead of e.(b) Calculate...
Question:
(a) Verify Equation 36.8 by considering a single-electron atom with nuclear charge Ze instead of e.(b) Calculate the ionization energies for single-electron versions of helium, oxygen, lead, and uranium.?
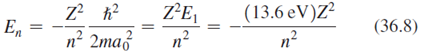
Transcribed Image Text:
Z²E, n? (13.6 eV)Z² n? (36.8) En n2 2ma
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
a Equation 368 is the formula for the ionization energy of an electron in an atom given by ...View the full answer

Answered By

Darwin Romero
I use a hands-on technique and am approachable to my students. I incorporate fun into my lessons when possible. And while my easy-going style is suitable for many subjects and grades, I am also able to adapt my style to the needs of the student. I can describe myself as friendly, enthusiastic and respectful. As a teacher, we can easily get respect from the students if they would feel respected first
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the ionization energy of the He+ ion in kJ/mol (this would be the second ionization energy of He). See Problem 8.107. The Bohr formula for the energy levels of an ion consisting of a...
-
If the outer electron in sodium moves in the n = 3 Bohr orbit, the effective nuclear charge would be Ze = 1e, and the energy of the electron would be 13.6 eV/3 2 = 1.51 eV. However, the ionization...
-
(a) Calculate the energies of an electron in the hydrogen atom for n = 1 and for n = . How much energy does it require to move the electron out of the atom completely (from n = 1 to n = ), according...
-
D. Prepare the consolidation worksheet journal entries to eliminate the effects of inter-entity transactions as at 30 June 2017. On 1 July 2013 Douglas Ltd acquired all of the share capital (cum...
-
(a) When propanol containing deuterium (D, or 2H; rather than hydrogen at the oxyger, CH3CH2CH2OD, is treated with an excess of H2O containing a catalytic amount of NaOH, 1-propanol is formed...
-
Once a week, Haziq rows his boat from the island where he lives to the mainland. The journey time, X minutes, is normally distributed with mean and variance 2 . a. Given that P(20 X < 30) = 0.32...
-
Good internal control procedures for cash include which of the following? (a) All cash disbursements, other than those for very small amounts, are made by check; (b) One employee counts cash received...
-
The standards for one case of Springfever Tonic are: Direct materials . . . . . . . . . . . . . . . . . . . . 6 lbs. @ $5.00/lb. = $30 Direct labor . . . . . . . . . . . . . . . . . . . . . . . 5...
-
Esperado Furnishings are retailers who purchase and sell household furnishings, including table lamps. The business uses a perpetual inventory system and adjusts cost of goods sold for any shortage...
-
Explain why errors in the valuation of inventory at the end of the year are sometimes called counterbalancing or self-correcting.
-
Form the radial probability density P 2 (r) associated with the 2s state of Equation 36.7, and find the electrons most probable radial position. 1 -r/2ao (36.7) 25 2 ao 4V2ma
-
Excimer lasers for vision correction generally use a combination of argon and fluorine to form a molecular complex that can exist only in an excited state. Stimulated de-excitation produces 6.42-eV...
-
Write the numeral as a Babylonian numeral. 23
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
the assessment include developing gantt chart, work breakdown structure and and all task 3 are related to its respective task 2. all the instructions are given in the assignment itself. Assessment...
-
Mens heights are normally distributed with mean 68.6in. and standard deviation 2.8in. Air Force Pilots The U.S. Air Force required that pilots have heights between 64 in. and 77 in. Find the...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
ACC1810 - PRINCIPLES OF FINANCIAL ACCOUNTING Project 11: Chapter 11 - Stockholders' Equity Part B: Financial Statements The accounts of Rehearsal Corporation are listed along with their adjusted...
-
Give an example to show that a b = a b.
-
Consider a closed, rigid tank with a volume of 0.8L, filled with cold water initially at 27C. The tank is filled such that there are no voids (air pockets) within. The initial pressure within the...
-
The rigid beam is supported by a pin at C and an A992 steel guy wire AB of length 6 ft. If the wire has a diameter of 0.2 in., determine the distributed load w if the end B is displaced 0.12 in....
-
The stressstrain diagram for a bone is shown, and can be described by the equation ε = 0.45(10 6 ) Ï + 0.36(10 12 ) Ï 3 , where s is in kPa. Determine the yield strength...
-
The stressstrain diagram for a bone is shown and can be described by the equation ε = 0.45(10 6 ) Ï + 0.36(10 12 ) Ï 3 , where Ï is in kPa. Determine the modulus of...
-
Year Cash Inflows 0 -1,000,000 1 100,000 2 400,000 3 500,000 4 300,000 5 100,000 Given this information: a) Compute the NPV of the investment in the firms cost of capital is 10% b) Compute the IRR.
-
Answer these questions based on tesla.If you had invested $ 5 , 0 0 0 on September 1 8 , what would be your gain ( loss ) be if you sold on November 1 4 ? Using the information in the annual report,...
-
Current Assets Listed here are certain accounts of Jenkins Company at the end of 2019: l'repare the current assets section of Jenkins's balance sheet at the end of 2019

Study smarter with the SolutionInn App


