(a) Suppose that a sealed, insulated container consists of two compartments, and that one of them is...
Question:
(a) Suppose that a sealed, insulated container consists of two compartments, and that one of them is filled with an ideal gas and the other is a vacuum. The partition separating the compartments is removed.
How does the temperature of the gas change? (Answer: it stays the same. Explain.) Obtain an expression for the final potential temperature, in terms of the initial temperature of the gas and the volumes of the two compartments.
Reconcile your answers with the first law of thermodynamics for an ideal gas, that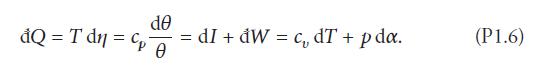
(b) A dry parcel that is ascending adiabatically through the atmosphere will generally cool as it moves to lower pressure and expands, and its potential temperature stays the same. How can this be consistent with your answer to part (a)?
Step by Step Answer:

Essentials Of Atmospheric And Oceanic Dynamics
ISBN: 9781107692794
1st Edition
Authors: Geoffrey K. Vallis





