Question: If you run a fuel cell in reverse as an electrolysis cell, H 2 O is split into its elementary gases, H 2 (g) and
If you run a fuel cell in reverse as an electrolysis cell, H2O is split into its elementary gases, H2(g) and = O2(g). This way you can make hydrogen and have it stored in case of the loss of outside electric power. The hydrogen can then be fed back to your electrolysis unit acting as a fuel cell. (1) If it takes 1.23 V to electrolyze water, what is the overall efficiency defined as (Theoretical Power Required in MW)/(Actual Power Required in MW) of this scheme? (2) If the electrolysis requires 1.50 V, what then is its overall efficiency?
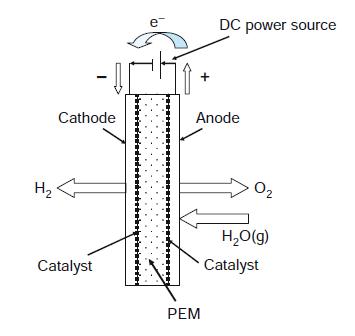
e DC power source Cathode Anode H2 O2 H,O(g) Catalyst Catalyst PEM H***************..**
Step by Step Solution
3.32 Rating (176 Votes )
There are 3 Steps involved in it
The overall efficiency of a fuel cellelectrolysis cell ... View full answer

Get step-by-step solutions from verified subject matter experts


