Question:
Consider the porous slab shown in the figure below. Very tiny pores of 20 A diameter run through the 2.0 cm slab in parallel array. This device will ultimately serve as a drug delivery vehicle for a drug that is soluble in ethanol. As part of the device development, we are interested in diffusion aspects of this unit with respect to ethanol and water containing no drug. The pores are initially filled with liquid ethanol. Ethanol (molecular weight 46 g/gmole) has an approximate molecular diameter of 4 A, and water (molecular weight 18 g/gmole) has an approximate molecular diameter of 3 A. The viscosity of liquid ethanol is 0.85 cP at 313 K. This ethanol-filled porous slab is placed in a large, well-mixed vat of liquid water at 313 K. Water diffuses into the ethanol-filled pores of the slab. After 10 min of contact time, what is the concentration of water 2.0 mm(0.2 cm) into the slab? It can be assumed that water does not penetrate very far into the pores of the slab, and that the surrounding liquid is essentially pure water at all times.
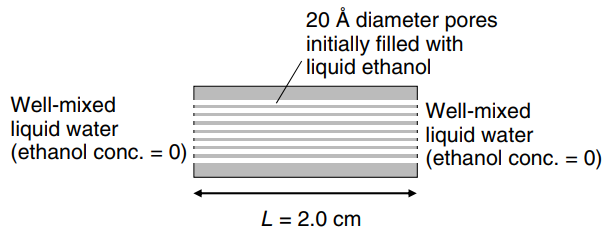
Transcribed Image Text:
20 Å diameter pores initially filled with / liquid ethanol Well-mixed liquid water (ethanol conc. = 0) Well-mixed liquid water = 0) (ethanol conc. %3D L = 2.0 cm








