Predict the shape of photometric titration curves (after correction for volume change) ifat the wavelength selectedthe molar
Question:
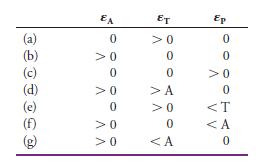
Predict the shape of photometric titration curves (after correction for volume change) if—at the wavelength selected—the molar absorptivities for the analyte A, the titrant T, and the product P are as follows:
Transcribed Image Text:
EA ET Ep (a) (b) (c) (d) (e) (f) (g) >0 >0 >0 >A >0 <Τ >0 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
7. Provide structures for these acid base reactions in the boxes and rationalize your answer using an arrow pushing mechanism. Predict whether each transformation is exothermic (Keq >1), endothermic...
-
Predict the shape of the nitronium ion, NO from its Lewis structure and the VSEPR model. It has one Raman active vibrational mode at 1400 cm-1, two strong IR active modes at 2360 and 540 cm-1, and...
-
Predict the shape of the distribution of the salaries of 25 chief executive officers (CEOs). A typical value is about 50 million per year, but there is an outlier a about 200 million. EXAMPLE 1...
-
Part A.: You are considering launching a strategic alliance with a competitor to join your separate skills to develop a new jointly owned technology. Both you and your partner have the option of...
-
How much more will you have in your RRSP 30 years from now if you make fixed contributions of $3000 at the end of each of the next 30 years, instead of waiting 15 years and making annual...
-
In a study of women science majors, the following data were obtained on two groups, those who left their profession within a few months after graduation (leavers) and those who remained in their...
-
Expected inflation is decided upon in the previous period. Explain why that would affect both current and future decisions.
-
One manager states, When a new system is proposed, I want a written report, not an oral presentation, which is like a sales pitch. I only want to see the facts about costs, benefits, and schedules....
-
Replace the Fair Value amounts provided in the question with the following: Cash $10,400 Accounts Receivable 23,100 Inventory 68,000 Trademarks 40,000 Patents 71,000 Current Liabilities 36,000...
-
The transactions completed by Fleetfoot Courier Company during December, the first month of the fiscal year, were as follows: Dec. 1. Issued Check No. 610 for December rent, $5,260. 2. Issued Invoice...
-
Why are fluorescence methods potentially more sensitive than absorption methods?
-
Mercury(II) forms a 1:1 complex with triphenyltetrazolium chloride (TTC) that exhibits an absorption maximum at 255 nm. The mercury(II) in a soil sample was extracted into an organic solvent...
-
Consider a molecule with fixed dipole \(\vec{d}\) in a time-dependent electric field \(\vec{E}(t)\). Calculate the Berry connection and Berry magnetic field in this case. Do we have a geometrical...
-
Why do you think it is important to consider only relevant costs when conducting a differential analysis for a major purchase? Why not consider all possible costs in your decision? provide specific...
-
How do power dynamics and influence tactics shape decision-making processes and organizational politics within hierarchical structures ?
-
How do I answer these given the information below? Loan Amount? Loan to Value? Loan to Cost? Payment amount? Loan Balance at Maturity? Given Information: Property Cost: $1,000,000 Bank Policy on LTV:...
-
In your initial post, first do the following: Use scholarly references to define Project Management (PM), Systems Development Life Cycle (SDLC), and Application Life Cycle (AL). Then, in the same...
-
How do concepts of diversity and inclusion vary across different cultural and geographical contexts, and what strategies can multinational organizations employ to navigate these variations...
-
Tally-Ho Horse Farms, Inc., began 2018 with cash of $170,000. During the year, Tally-Ho earned service revenue of $595,000 and collected $590,000 from customers. Expenses for the year totaled...
-
Rowland Textile Inc. manufactures two products: sweatshirts and T-shirts. The manufacturing process involves two activities: cutting and sewing. Expected overhead costs and cost drivers are as...
-
The boiling points of HCl and CO2 are nearly the same (285C and 278C). Explain why CO2 can be removed from an aqueous solution by boiling briefly while essentially no HCl is lost even after boiling...
-
The digestion of a 0.1417-g sample of a phosphorus-containing compound in a mixture of HNO3 and H2SO4 resulted in the formation of CO2, H2O, and H3PO4. Addition of ammonium molybdate yielded a solid...
-
Neohetramine, C16H21ON4 (285.37 g/mol), is a common antihistamine. A 0.1247-g sample containing this compound was analyzed by the Kjeldahl method. The ammonia produced was collected in H3BO3-; the...
-
Calculate the current ratio and the quick ratio for the following partial financial statement for Tootsie Roll Note: Round your answers to the nearest hundredth
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Golden Corporation's current year income statement, comparative balance sheets, and...
-
Glencove Company makes one model of radar gun used by law enforcement officers. All direct materials are added at the beginning of the manufacturing process. Information for the month of September...

Study smarter with the SolutionInn App


