Describe the reactions that convert epinephrine to the compound shown here. CH3 O OH 0
Question:
Describe the reactions that convert epinephrine to the compound shown here.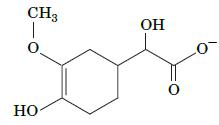
Transcribed Image Text:
CH3 O OH 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The compound shown is vanillylmandelic acid VMA which is a metabolite of epinephrine Epinephrine is ...View the full answer

Answered By

Sarah Khan
My core expertise are:
-_ Finance
-_ Business
-_ Management
-_ Marketing Management
-_ Financial Management
-_ Corporate Finance
-_ HRM etc...
I have 7+ years of experience as an online tutor. I have hands-on experience in handling:
-_ Academic Papers
-_ Research Paper
-_ Dissertation Paper
-_ Case study analysis
-_ Research Proposals
-_ Business Plan
-_ Complexed financial calculations in excel
-_ Home Work Assistance
-_ PPT
-_ Thesis Paper
-_ Capstone Papers
-_ Essay Writing etc...
5.00+
91+ Reviews
92+ Question Solved
Related Book For 

Fundamentals Of Biochemistry Life At The Molecular Level
ISBN: 9781118918401
5th Edition
Authors: Donald Voet, Judith G Voet, Charlotte W Pratt
Question Posted:
Students also viewed these Sciences questions
-
Describe the reactions that are used in the steam-reforming process for the production of hydrogen.
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
Epinephrine (adrenalin; see also Chapter 6 Opening) is produced in your body in a two-step process that accomplishes the transfer of a methyl group from methionine (Problem 70) to norepinephrine (see...
-
$ 45.00 Direct material: 5 pounds at $9.00 per pound Direct labor: 3 hours at $14 per hour Variable overhead: 3 hours at $9 per hour Total standard variable cost per unit 42.00 27.00 $114.00 The...
-
Why is the server segment of the computer industry becoming so competitive today? SGI, or Silicon Graphics International, was formed by the merger of Rackable Systems and Silicon Graphics in May...
-
9. Prepare the partial balance sheet at December 31, 2019, and show the net realizable value of accounts receivable assuming % of accounts receivable method. Accounts Receivable $ 270,000 Less:...
-
5. Suppose that Xn E R. (a) Prove that {xn} is bounded if and only if there is a C > such that IXn I :s; C for all n E N. (b) Suppose that {xn} is bounded. Prove that xnlnk -+ 0, as n -+ 00, for all...
-
The Fraser Paper Company produces large rolls of white paper weighing 1,000 kilograms for wholesalers for $1,500 each. The wholesalers then cut the paper into standard-sized sheets and package it in...
-
23) Jobcoating A) records the flow of costs for each product or service 3) cannot be used by the service industry allocates an equal amount to each unit made during D) is used when each wit o utput...
-
From which amino acid is 2-phenylethanol derived and what chemical changes take place in the conversion? OH 2-Phenylethanol
-
Many of the most widely used herbicides inhibit the synthesis of aromatic amino acids. Explain why the compounds are safe to use near animals.
-
It can be said that complex IS projects commonly fail because organizations not only fail to learn, but also learn to fail. Explain how this happens. What should an organization do to avoid this...
-
the assessment include developing gantt chart, work breakdown structure and and all task 3 are related to its respective task 2. all the instructions are given in the assignment itself. Assessment...
-
Mens heights are normally distributed with mean 68.6in. and standard deviation 2.8in. Air Force Pilots The U.S. Air Force required that pilots have heights between 64 in. and 77 in. Find the...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
ACC1810 - PRINCIPLES OF FINANCIAL ACCOUNTING Project 11: Chapter 11 - Stockholders' Equity Part B: Financial Statements The accounts of Rehearsal Corporation are listed along with their adjusted...
-
Match the term to the description. Outcome evaluation Focuses on the accomplishments and impact of a service, program, or policy and its effectiveness in attaining its outcomes set prior to...
-
Explain the phrase price-led costing?
-
As you rewrite these sentences, replace the cliches and buzzwords with plain language (if you don't recognize any of these terms, you can find definitions online): a. Being a jack-of-all-trades, Dave...
-
Muscalure is the sex pheromone of the common housefly and has the molecular formula C 23 H 46 . When treated with O 3 followed by DMS, the following two compounds are produced. Draw two possible...
-
Propose a plausible mechanism for each of the following reactions: a. b. stitl. [H,SO,] Conc. H2SO4
-
Suggest an efficient synthesis for the following transformation: CI H
-
Now determine the total (net) energy absorbed for each land cover type. (12 marks) Snow Grass Water Asphalt 0100h 0.0 0200h 0.0 0300h 0.0 0400h 0.0 0500h 0.0 0600h 2.4 0700h 23.5 0800h 86.4 0900h...
-
There is a bond on a companys books with an original term of 10 years that was purchased for a premium at its issuance, just over 2 years ago. The bond pays semi-annual interest. With the receipt of...
-
Instructions: The Accounting Department has provided you with two reports from the first quarter (Q1) of the fiscal year: the General Ledger (GL) Detail report, and the Income Statement. Use both of...

Study smarter with the SolutionInn App


