Use Coulombs law (Equation 2-1) to explain why a salt crystal such as NaCl remains intact in
Question:
Use Coulomb’s law (Equation 2-1) to explain why a salt crystal such as NaCl remains intact in benzene (C6H6) but dissociates into ions in water.
Data from Equation 2-1:
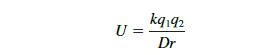
Transcribed Image Text:
U = kq192 Dr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The formula is Coulombs law which states that the force between two charged particles is proportional to the product of their charges and inversely pr...View the full answer

Answered By

Aun Ali
I am an Associate Member of Cost and Management Accountants of Pakistan with vast experience in the field of accounting and finance, including more than 17 years of teaching experience at university level. I have been teaching at both undergraduate and post graduate levels. My area of specialization is cost and management accounting but I have taught various subjects related to accounting and finance.
5.00+
13+ Reviews
32+ Question Solved
Related Book For 

Fundamentals Of Biochemistry Life At The Molecular Level
ISBN: 9781118918401
5th Edition
Authors: Donald Voet, Judith G Voet, Charlotte W Pratt
Question Posted:
Students also viewed these Sciences questions
-
Use Figure 7.1 to explain why a rise in the price of an input must increase the total cost of producing any given output level. What does this result suggest about how such a price increase shifts...
-
In Problems 5 and 6, use Theorem 1 to explain why no maxima or minima exist. Maximize f(x,y) = 6x + 5y + 24 subject to 3x + 2y = 4
-
Use the results of Problem 7 to explain why a deficiency of GLUT2 produces symptoms resembling those of Type 1 glycogen storage disease (Box 16-2).
-
Lombard Ltd has been offered a contract for which there is available production capacity. The contract is for 20,000 identical items, manufactured by an intricate assembly operation, to be produced...
-
With interest rates at historic lows in the United States, what is the effect on the optimal rate of extraction for a Texas oilfield owner? Explain the intuition that supports your answer.
-
There is an old saying, What I hear I forget, what I see I can understand and what I do I know. What is the relevance of this to presentation? LO1
-
The process can be applied for more than two Brownian motions.
-
Using the Morningstar information in Exhibit 13.10, evaluate the performance of the QQQ index-based ETF. Specifically, comment on how well it tracks the underlying NASDAQ 100 index and how its...
-
Required information Problem 1-2A Computing missing information using accounting knowledge LO A1, P1 The following information applies to the questions displayed below.] The following financial...
-
(a) Would phosphoric acid or succinic acid be a better buff er at pH 5? (b) Would ammonia or piperidine be a better buff er at pH 9? (c) Would HEPES or Tris be a better buff er at pH 7.5?
-
(a) At any instant, how many water molecules are ionized in 1 L of pure water at pH 7.0? (b) Express this number as a percentage of the total water molecules.
-
The_______ are the endpoints of the transverse axis of a hyperbola.
-
Silver Company makes a product that is very popular as a Mothers Day gift. Thus, peak sales occur in May of each year, as shown in the companys sales budget for the second quarter given below: April...
-
Among the following statements, select the ones which have a positive environmental impact. Choose several answers Minimising the impact of a product on the environment Avoiding the destruction of a...
-
Developing Financial Statements: All organizations, including those in the healthcare industry, need to make money to be profitable and survive. Financial statements, such as balance sheets, profit...
-
The engineers estimated that on average, fuel costs, assuming existing routes and number of flights stay the same, would decrease by almost 18% from an average of 42,000 gallons of jet fuel per...
-
It's the latest Berkeley trend: raising chickens in a backyard co-op coop. (The chickens cluck with delight at that joke.) It turns out that Berkeley chickens have an unusual property: their weight...
-
Weber Industries has three activity cost pools and two products. It expects to produce 3,000 units of Product BC113 and 1,400 of Product AD908. Having identified its activity cost pools and the cost...
-
Why should you not model a decision variable as a random variable with a probability distribution?
-
Which of the following polypeptides is most likely to form an helix? (a) CRAGNRKIVLETY (b) SEDNFGAPKSILW (c) QKASVEMAVRNSG
-
The X-ray crystallographic analysis of a protein often fails to reveal the positions of the first few and/or the last few residues of a polypeptide chain. Explain.
-
You are performing site-directed mutagenesis to test predictions about which residues are essential for a protein's function. Which of each pair of amino acid substitutions listed below would you...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


