a) Calculate the enthalpy, entropy, and volume of a solution that contains 37.5% by mole heptane in
Question:
a) Calculate the enthalpy, entropy, and volume of a solution that contains 37.5% by mole heptane in decane, at 15 °C, 1 bar.
b) Calculate the amount of heat needed to raise the temperature of the solution to 40 °C under constant pressure of 1 bar.
c) Calculate the entropy change of the solution in part b.
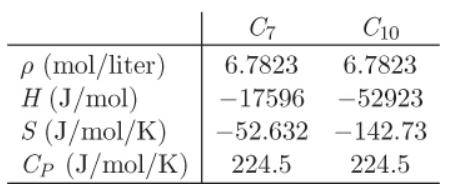
Additional data: You may assume that the components form an ideal solution. The following properties of the pure components are known:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:





