An experiment on the vapor-liquid equilibrium for the methanol (1) + dimethyl carbonate (2) system at 337.35
Question:
An experiment on the vapor-liquid equilibrium for the methanol (1) + dimethyl carbonate (2) system at 337.35 K provides the following information (S. Yunhai et al., 2005):
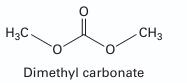
x1 50.0, y1 50.0 and P = 41.02 kPa.
x1 50.20, y1 50.51 and P = 68.23 kPa.
x1 51.0, y1 51.0 and P = 99.91 kPa.
Use this information to estimate the system pressure and vapor-phase mole fraction when x1 = 0.8. Use the 1-parameter Margules equation.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





