Resolve Example Problem 11.1, but now include the nitrogen gas into the system. Note the Henrys Law
Question:
Resolve Example Problem 11.1, but now include the nitrogen gas into the system. Note the Henry’s Law constants for nitrogen in water in Table P11-15:

Transcribed Image Text:
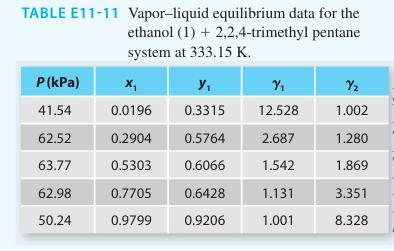
TABLE E11-11 Vapor-liquid equilibrium data for the ethanol (1) + 2,2,4-trimethyl pentane system at 333.15 K. P (kPa) 41.54 62.52 63.77 62.98 50.24 x₁ Y₁ 0.0196 0.3315 0.2904 0.5303 0.7705 0.9799 0.5764 0.6066 0.6428 0.9206 Y₁ 12.528 2.687 1.542 1.131 1.001 Y₂ 1.002 1.280 1.869 3.351 8.328
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The Henrys law constant for O 2 in water at 25C is given in Table 13.2. Which of the following is a reasonable constant when the temperature is 50C? Explain the reason for your choice. (a) 6.7 10 4...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
In Exercises 7681, find the domain of each function. g(x) = 4 x - 7
-
The Coca-Cola Company is a global soft drink beverage company (ticker symbol = KO) that is a primary and direct competitor with PepsiCo. The data in Exhibits 12.1312.15 include the actual amounts for...
-
According to the Pew Internet & American Life Project, 75% of American adults use the Internet (Pew Internet website, April 19, 2008). The Pew project authors also reported on the percentage of...
-
CURRENT ASSETS INVESTMENT POLICY Rentz Corporation is investigating the optimal level of current assets for the coming year. Management expects sales to increase to approximately $2 million as a...
-
Sachs Brands' defined benefit pension plan specifies annual retirement benefits equal to: 1.6% service years final year's salary, payable at the end of each year. Angela Davenport was hired by...
-
conomic releases economic indical Which economic indicator is most directly linked to the average person's cost of living? Nonfarm payrolls PMI GDP
-
Which system provided here, if any, would be best modeled by an ideal solution? If any of the solutions are non-ideal, discuss whether the Scatchard Hildebrand approach would be appropriate to model...
-
An experiment on the vapor-liquid equilibrium for the methanol (1) + dimethyl carbonate (2) system at 337.35 K provides the following information (S. Yunhai et al., 2005): x 1 50.0, y 1 50.0 and P =...
-
Matilda Moore has $21,000 of investment interest expense and $7,000 of net investment income in 2019. How much of the investment interest expense is deductible for tax purposes in 2019?
-
What strategies do you employ to mediate conflicts within a team to ensure that disagreements are resolved constructively and synergistically?
-
What goal(s) do you think the communication was intended to achieve? What type of promotional communication is it and why? What do you believe to be the advertising theme or central idea of the...
-
What Do You Know About Amazon Associate Program? What Would You Do To Increase Your Earnings With Amazon Associate Program? Is Affiliate Marketing And Referral Marketing One And The Same? What is...
-
As a leader, what do you think are important elements of a leadership team made up of those senior people that you will surround yourself with? Do you have (or have you had) a mentor? If so, how have...
-
What role do interorganizational relationships and alliances play in achieving strategic goals, and how do organizations manage these relationships to ensure mutual benefit and minimize risks ?
-
Which of the following ethers would be obtained in greatest yield directly from alcohols? CH1 CH3OCCH3 CH3 CH,, CH CH20CH2CH2CH3
-
As economic conditions change, how do banks adjust their asset portfolio?
-
A solution contains 63 different conjugate acid-base pairs. Among them is acrylic acid and acrylate ion, with the equiliborium ratio [acrylate]/[acrylic acid] = 0.75. What is the pH of the solution?...
-
Find the pH of a solution prepared by dissolving 1.00 g of glycine amide hydrochloride (Table 8-2) plus 1.00 g of glycine amide in 0.100 L. Glycine amide C;H&N20 H,N. FM 74.08 NH,
-
(a) Find the pH of a solution prepared by dissolving 1.00 g of glycine amide hydrochloride (Table 8-2) plus 1.00 g of glycine amide in 0.100 L. (b) How many grams of glycine amide should be added to...
-
A health insurance policy pays 70% of physical therapy costs after a deductible of $300. In contrast, an HMO charges $20 per visit for physical therapy. How much would a person save with the HMO of...
-
The income statement for the current year is as follows: Sales Cost of goods sold Gross profit Operating expenses: Depreciation expense: Other operating expense Total operating expense Operating...
-
.. yy This is a very simple and inexpensive method. However, it is not precise. Its quality heavily depends on the experience and ability of the buyer to judge the situation. As compared to other...

Study smarter with the SolutionInn App


