Consider the case of diffusion and reaction inside a slab. Initially the concentration of solute in the
Question:
Consider the case of diffusion and reaction inside a slab. Initially the concentration of solute in the slab is \(c_{a o}\). The slab is made of a material that when heated catalyzes the reaction of \(a\) so that \(a\) decomposes with first-order kinetics and a reaction rate constant, \(k^{\prime \prime}\). A heating fluid bathes the slab so that at the outer surfaces, the concentration of \(a\) is always 0 . Determine the concentration profile within the slab as a function of time so that we will know when the reaction is essentially complete and we can remove the slab from the heating fluid (Figure P6.26).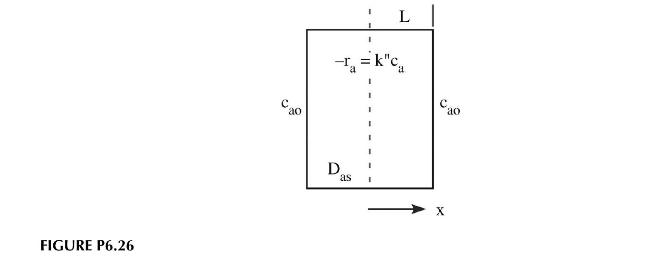
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





