Consider the continuous adsorption situation shown in the Figure P4.37. The adsorbate is present in the gas
Question:
Consider the continuous adsorption situation shown in the Figure P4.37. The adsorbate is present in the gas phase at a concentration, \(c_{a o}\). It adsorbs uniformly on the surface of the adsorbent and then diffuses through the liquid phase. Different adsorption isotherms are in common use including the Langmuir \(q=q_{o}\left(\frac{c_{a g}}{K_{a}+c_{a g}}\right)\) and Freundlich \(q=K_{a}\left(c_{a g}\right)^{n}\) isotherms. Here \(q\) represents the moles of adsorbate adsorbed per gram of adsorbent. \(q_{o}, n\), and \(K_{a}\) are constants that must be measured experimentally. Using each of these isotherms and assuming \(K_{e q}=1\) :
a. Solve for the concentration profile of adsorbate in the liquid phase.
b. Derive an expression for the mass transfer coefficient based on the concentration driving forces: \(\left(c_{e}-c_{a L}\right) ;\left(c_{a o}-c_{a L}\right)\).
Here \(c_{e}\) is the concentration of adsorbate in the adsorbent. You may assume the adsorbent has a mass, \(m_{o}\), a density, \(ho_{o}\), a molecular weight, \(M_{w o}\), and a volume, \(v_{o}\).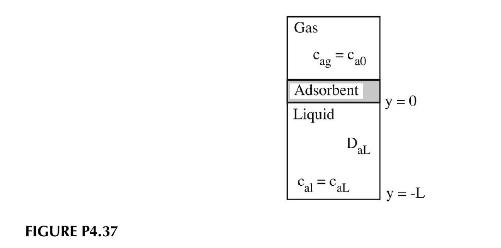
Step by Step Answer:






