Consider the R-245fa (1) + n-pentane (2) system in Figure 13-13. At the different state points (black
Question:
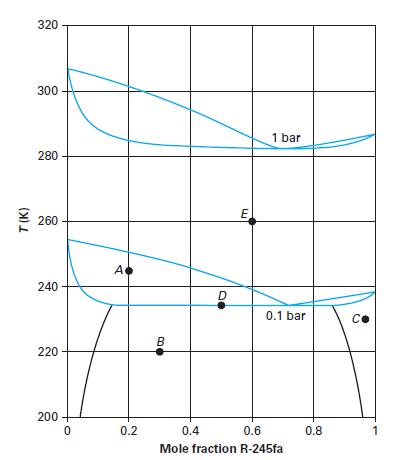
Consider the R-245fa (1) + n-pentane (2) system in Figure 13-13. At the different state points (black dots) provided on Figure 13-13, name the equilibrium phases and the compositions of those equilibrium phases. Note that the overall mixture composition is given using the symbol w.
Point A: T = 245 K; P = 0.1 bar; w1 = 0.2
Point B: T = 220 K; P = 0.1 bar; w1 = 0.3
Point C: T = 228 K; P = 0.1 bar; w1 = 0.97
Point D: T = 234 K; P = 0.1 bar; w1 = 0.5
Point E: T = 260 K; P = 0.1 bar; w1 = 0.6
Figure 13-13
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





