Hot coffee is contained in a cylindrical thermos bottle that is of length (L=0.3 mathrm{~m}) and is
Question:
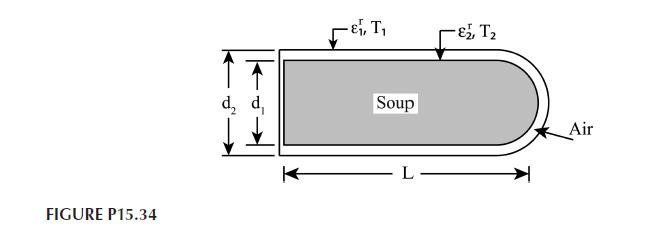
Hot coffee is contained in a cylindrical thermos bottle that is of length \(L=0.3 \mathrm{~m}\) and is laying its side as shown in Figure P15.34. The coffee container consists of a glass flask of diameter \(d_{1}=0.07 \mathrm{~m}\) separated from an aluminum housing of diameter \(d_{2}=0.08 \mathrm{~m}\) by air at atmospheric pressure. The outer surface of the flask and the inner surface of the housing are silver coated to provide emissivities of \(\varepsilon_{1}^{r}=\varepsilon_{2}^{r}=0.25\).
a. If these surface temperatures are \(T_{1}=75^{\circ} \mathrm{C}\) and \(T_{2}=35^{\circ} \mathrm{C}\), what is the heat loss from the coffee? Consider all relevant mechanisms.
b. How much would the heat loss be increased/reduced if the air space were evacuated?
c. How much would the heat loss be increased/reduced if the container were standing on its end (vertical)?
Step by Step Answer:






