Shown below is an energy level diagram and the emission line spectrum that arises from these energy
Question:
Shown below is an energy level diagram and the emission line spectrum that arises from these energy levels. What electron transition (drop) gave rise to the line labeled B in the emission spectrum?
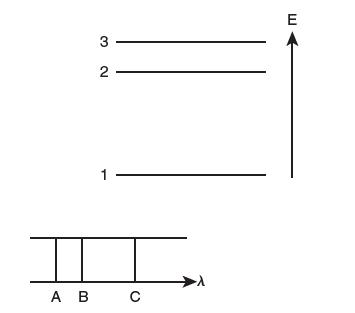
Transcribed Image Text:
I 3- N W 2- 1- A B C E
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
In an emission line spectrum each line corresponds to a specific electron transition between differe...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The new storage facilities of Menty Ltd are a bit disorganised at the moment and warehouse staff seem to wasting a lot of time transferring stock between premises. At times they have been unable to...
-
The visible lines in the hydrogen atom emission spectrum arise from transitions with a final state with n = 2. In what spectral region should we expect to find transitions that have a final state of...
-
8. The width of the Data Bus determines: a. The speed of data transfer b. The number of bits that can be transferred in parallel c. The physical length of the bus d. The clock frequency of the CPU [1...
-
Solve the problem. Jake Dowell has total fixed monthly expenses of $1367 and his gross monthly income is $3861. What is his debt-to-income ratio? Round to the nearest percent. ? 3% o 4% o 28% o 35%
-
Which budget must be prepared before the others? Why?
-
Explain the meaning of the following terms: a. Canceled check b. Outstanding check c. Deposit in transit d. Debit memorandum e. Credit memorandum f. Dishonored check g. Blank endorsement h. Deposit...
-
What is foreign exchange risk? How does a company become exposed to foreign exchange risk? LO9
-
Exodus Limousine Company has $1,000 par value bonds outstanding at 10 percent interest. The bonds will mature in 50 years. Compute the current price of the bonds if the percent yield to maturity is:...
-
Currington Company wants to use absorption cost-plus pricing to set the selling price on a newly remodeled product. The company plans to invest $164,000 in operating assets to produce and sell 12,000...
-
How would you describe the shape of the water and ozone molecules? Follow the convention! Ignore the lone pairs on each central atom. Cover them up and use a word or phrase that best describes the...
-
Arrange the following atoms in order of increasing atomic size: Ne, As, Se, K, S, Cl
-
Zeta Corporation reports the following results for Year 1 and Year 2: The adjusted taxable income is before Zeta claims any charitable contributions deduction, NOL or capital loss carryback,...
-
2.11.2Project:Performance Task: The Parallax Problem Project Geometry Sem 1 (S3537251) Julio Duenas Points possible:120 Date: ____________ The Scenario:You're looking for a sponsor to pay for you to...
-
If the most common treatment of assigning overapplied overhead was used, the final balance in Cost of Goods Sold would have been * (1 Point) At the end of the last fiscal year, BREAD Company had the...
-
Angelina received new word processing software for her birthday. She also received a cheque with which she intends to purchase a new computer. Angelina's UNILUS Professor assigned a paper due in two...
-
At date t, the portfolio P to be hedged is a portfolio of Treasury bonds with various possible maturities. Its characteristics are as follows: Value YTM MD Convexity $1,450 6% 4.25 55 We consider...
-
A playground merry-go-round with an axis at the center (radius R = 1.3 m and rotational inertia | = 1.2 x 103 kgm2) is initially rotating at angular velocity w = 0.21 rad/s clockwise). A girl of mass...
-
Prove that ||r(t)|| is constant if and only if r(t) r'(t) = 0.
-
Juarez worked for Westarz Homes at construction sites for five years. Bever was a superintendent at construction sites, supervising subcontractors and moving trash from sites to landfills. He...
-
Why is maintaining the entanglement of pairs A and B and A and X the crucial ingredient of teleportation?
-
Why is it not possible to reconstruct the wave function of a quantum mechanical superposition state from experiments?
-
Why does the relative uncertainty in x for the particle in the box increase as n ?
-
This short exercise demonstrates the similarity and the difference between two ways to acquire plant assets. (Click the icon to view the cases.) Compare the balances in all the accounts after making...
-
Balance sheet and income statement data for two affiliated companies for the current year appear below: BALANCE SHEET As at December 31, Year 6 Albeniz Bach Cash $ 40,000 $ 21,000 Receivables 92,000...
-
please reference excel cells Caroll Manufacturing company manufactures a single product. During the past three weeks, Caroll's cost accountant observed that output costs varied considerably. The...

Study smarter with the SolutionInn App


