In the process below, all streams are mixtures of methane/carbon monoxide with molar compositions indicated on the
Question:
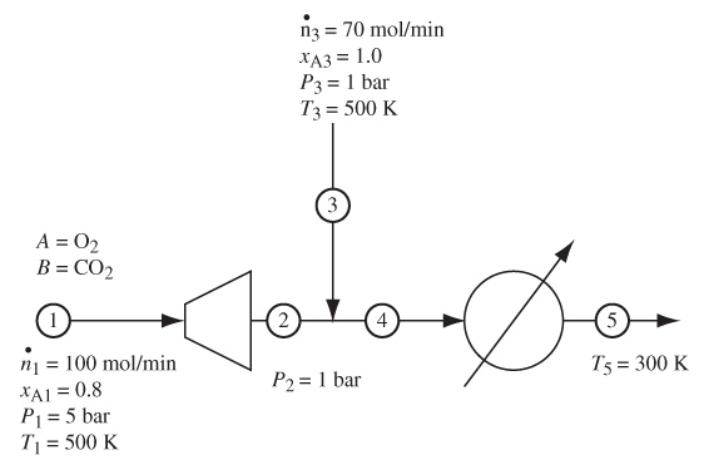
In the process below, all streams are mixtures of methane/carbon monoxide with molar compositions indicated on the flow chart in Figure 9-3. Calculate the material and energy balances and perform an entropy analysis of the mixing point, the heat exchanger, and of the entire process. The heat capacities of methane and carbon monoxide are CPA = 40.8 J/mol K, and CPB = 29.4 J/mol K, respectively.
Figure 9-3.
Transcribed Image Text:
A = 0₂ B = CO₂ 1 n₁ = 100 mol/min XA1 = 0.8 P₁ = 5 bar T₁ = 500 K 2 n3 = 70 mol/min XA3 = 1.0 P3 = 1 bar T3 = 500 K 3 o 4 P₂ = 1 bar (5) T5 = 300 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
Material Balances Mixing Point The total number of moles of methane and carbon monoxide in the mixing point stream is ntotal nA1 nB1 100 molmin 70 mol...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:
Students also viewed these Engineering questions
-
You have been assigned to simulate a flash evaporator that separates a Liquid feed stream containing benzene and toluene at temperature T F (?C) into liquid and vapor product streams in equilibrium...
-
The wastewater treatment plant at the Ossabaw Paper Company paper mill generates about 24 tonnes of sludge per day. The consistency of the sludge is 35%, meaning that the sludge contains 35 wt%...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following linear systems Ax = b have x as the actual solution and x as an approximate solution. Compute ||x x|| and ||Ax b|| a. 1/2 x1 + 1/3 x2 = 1/63, 1/3 x1 + 1/4 x2 = 1/168, x = ( 1/7 ,1/6)t...
-
Hotel rooms in Small town go for $100, and 1,000 rooms are rented on a typical day. a. To raise revenue, the mayor decides to charge hotels a tax of $10 per rented room. After the tax is imposed, the...
-
On June 1, two jobs were in process at Spiffy Painters, Inc. Details of the jobs follow: Direct Direct Job No. Material Labor LP Rae ae ine oa 8 oe cn Ree $174 $64 PeaNE cn Sate pO uence cuore cen...
-
What do you think about Subways method and level of compensating its master franchisee and regular franchisees in China? Is the method satisfactory? Is there room for improvement? LO.1
-
The adjusted trial balance of Harmony Company at December 31, 2010, includes the following accounts: S. Harmony, Capital $15,600; S. Harmony, Drawing $6,000; Service Revenue $35,400; Salaries Expense...
-
A printing company purchases a new printer for $15,000 with a 5-year useful life. Their accountant informs them that they must use the Modified Accelerated Cost Recovery System (MACRS) to calculate...
-
Streams A and B are mixed adiabatically to produce stream C. Stream A is at 1 bar, 50 C, it contains pure methane and its flow rate is 0.2 mol/min. Stream B is at 1 bar, 100 C, contains a...
-
Air is compressed from 1 bar, 25 C to 50 bar, and subsequently is cooled to 300 K using cooling water. Assuming air to be an ideal-gas mixture and the compressor to be 100% efficient, calculate the...
-
As the cost accounting manager at Cambria Chemicals (CC), you are responsible for compiling and reporting various performance measures to the senior managers. The company instituted many efficiency...
-
1. Mainland purchased a machine for $85,000 on 1 January 20x7 and assigned it a useful life for 10 years. On 31 March 20x9 it was revalued to $93,000 with no change in useful life. Complete the table...
-
Find the equation of the regression line and identify a characteristic of the data that is ignored by the regression line X 10 8 13 9 11 14 6 4 12 7 5 Y 7.46 6.77 12.74 7.11 7.81 8.84 6.08 5.39 8.15...
-
For each of the following independent cases, fill in the missing amounts in the table: (Indicate the effect of each variance by selecting "F" for favorable, "U" for unfavorable.) Case Direct Labor...
-
All views expressed in this paper are those of the authors and do not necessarily represent the views of the Hellenic Observatory or the LSE George Alogoskoufis Greeces Sovereign Debt Crisis:...
-
Current Attempt in Progress Nash Company is constructing a building. Construction began on February 1 and was completed on December 31. Expenditures were $1,812,000 on March 1, $1,212,000 on June 1,...
-
Obtain a copy of an entity's Letter of Transmittal and MD&A from its CAFR. Analyze. a. Summarize and describe the nature of the information in the Letter of Transmittal. Does it fundamentally differ...
-
The following items were displayed in the statement of affairs for Lubbock Company: Fully secured liabilities ......... $90,000 Partially secured liabilities ....... 12,000 Unsecured liabilities...
-
A stream of liquid nitrogen enters an adiabatic, steady-state valve as a saturated liquid at P = 2 MPa. The material leaves the valve at P = 0.6 MPa. Use the data in Figure 2-3 to determine the...
-
Prove that the process shown in Figure 4-9 is impossible if the cylinder contains a monatomic ideal gas P = 1 bar, T= 300 K P = 1.0 bar T = 300 K l l l l l l l l l P= 1 bar, T = 300 K P = 5.0 bar T =...
-
A 10 ounce glass of water (half full) is initially at 15C. It is left outside overnight in a location where the air temperature is 5C. By morning the glass is in equilibrium with the surroundings....
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


