Methanol at 25 C, 8 bar is heated under constant P in closed system. a) Determine b)
Question:
Methanol at 25 °C, 8 bar is heated under constant P in closed system.
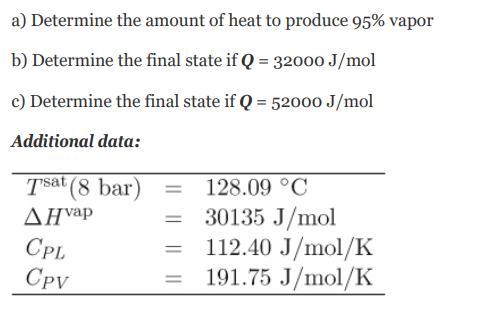
Transcribed Image Text:
a) Determine b) Determine the final state if Q = 32000 J/mol c) Determine the final state if Q = 52000 J/mol Additional data: the amount of heat to produce 95% vapor Tsat (8 bar) AH vap CPL CPV 128.09 C 30135 J/mol 112.40 J/mol/K = 191.75 J/mol/K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
a To determine the amount of heat to produce 95 vapor we can use the following equation Q nv Hvap nl CpLTf Ti where Q is the heat required J nv is the number of moles of vapor mol Hvap is the heat of ...View the full answer

Answered By

Saista Firdous Ansari
I worked for Chegg as a subject matter expert in operation management for 3 years.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:
Students also viewed these Engineering questions
-
Clear Strategy Corp., a strategic marketing consulting firm, began operations on January 1, 2016. Its post-closing trial balance at December 31, 2016, and 2017, is shown below along with some other...
-
Give the advantages of parseval's power theorem and rayleigh's energy theorem in communication system
-
Water at 20 C 30 bar is heated in a closed system under constant pressure. a) If the quality in the final state is 75%, what is the amount of heat? b) If the amount of heat is 2000 kJ/kg, what is the...
-
Using the case study, characterize Amazons approach to marketing communications.
-
A start-up company that makes hydraulic seals borrowed $800,000 to expand its packaging and shipping facility. The contract required the company to repay the investors through an innovative mechanism...
-
Devise an observation schedule of your own for observing an area of social interaction in which you are regularly involved. Ask people with whom you normally interact in those situations how well...
-
What common pricing practices are considered to be illegal or unethical?
-
Drake Paper Company sells on terms of net 30. The firms variable cost ratio is 0.80. a. If annual credit sales are $20 million and its accounts receivable average 15 days overdue, what is Drakes...
-
Assume that Shannons decides to move forward with its loyalty / rewards program. Estimates for the cost per customer are $5.96 per month. Average customer margins, before subtracting off the cost of...
-
a) Obtain the heat of vaporization of steam at 30 bar. b) Saturated liquid water at 30 bar is heated until the quality is 75%. What is the amount of heat? c) Saturated liquid water at 30 bar is...
-
Wet steam at 200 C with 80% moisture is heated by removing 600 kJ/kg of heat. a) Determine the final state (pressure and temperature and quality, if a two-phase system) if cooling is at constant...
-
On September 1, 2022, The Shoppes at Forest Lake, Inc., purchased inventory costing $63,000 by signing an 8%, six-month, short-term note payable. The company will pay the entire note (principal and...
-
You are the cost accountant of an engineering concern which has three departments - preparation, machining and assembly. The budgeted direct labour hours for the workshops are 8,000, 12,000 and...
-
What alternative to fostering fun and enjoyment at work do you think might have worked for Zappos?
-
Using the techniques of dimensional analysis, and assuming that experimentation shows the dimensionless number to be 1, derive the following equation: E v = Job card two The results of an ultrasonic...
-
Given the historical cost of product Carla Vista is $13, the selling price of product Carla Vista is $15, costs to sell product Carla Vista are $3, the replacement cost for product Carla Vista is...
-
What causes of outliers in statistics and when I create a boxplot why do I not see the outliers. What steps are to take in creating a boxplot?
-
In Figland Company's first year of operations (2017), the company had pre-tax book income of $500,000 and taxable income of $800,000. Figland's only temporary difference is for accrued product...
-
Use multiplication or division of power series to find the first three nonzero terms in the Maclaurin series for each function. y = e x2 cos x
-
This problem expands upon Example 15-4. A reaction vessel is rigid and has a volume of 500 L and initially contains 10 moles of o-xylene. The liquid phase is exposed to catalyst that facilitates...
-
For an ethylene glycol n-butyl ether (1) + water (2) system at 310 K with 70% by mass water, determine if the system is one stable liquid phase or two stable liquid phases at equilibrium. If the...
-
30 mol/s of hydrogen gas and 15 mol/s of air, each compressed to 25 bar, enter a steady state reactor as shown in Figure 15-5, where the nitrogen in the air reacts with the hydrogen to form ammonia:...
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


