Starting from the expression of the total energy carried by the lattice vibrations in Eq. (6.60), show
Question:
Starting from the expression of the total energy carried by the lattice vibrations in Eq. (6.60), show that the heat capacity Cv = (dE/dT)v can be written as: 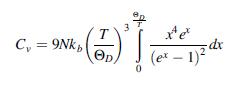
Transcribed Image Text:
T C, = 9Nk, Op (et – 1)2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
The total energy carried by the lattice vibrations can be expressed as E 9NkB T3 qpT2 0DT x3ex ...View the full answer

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Show that van der Waals equation can be written as a cubic equation in the compressibility factor involving the reduced pressure and reduced temperature as (27 P 27 P2 512 T, (T+1) z2- - 1) z - Z3 ST...
-
Show that the secular equation (9.82) can be written as
-
Show that the group velocity can be written as dv = v - di Og
-
Pepsin is the principal digestive enzyme of gastric juice. A 1.40 g sample of pepsin is dissolved in enough water to make 4.50 mL of solution. The osmotic pressure of the solution is found to be...
-
Fellowes and Associates Chartered Accountants is a successful mid-tier accounting firm with a large range of clients across Canada. In 2011, Fellowes and Associates gained a new client, Health Care...
-
Discuss three common examples of natural processes that involve an increase in entropy. Be sure to account for all parts of each system under consideration.
-
3. Assuming that this hedge relationship qualifies for hedge accounting: a. Determine the estimated fair value of the hedge at December 31, 2016. Recall that the hedge contract is in effect for the...
-
The information presented below is for MedQuest Pharmacy, Inc. a. Salaries for the period December 26, 2012, through December 31, 2012, amounted to $17,840 and have not been recorded or paid. (Ignore...
-
1. CREATE JOURNAL ENTRIES FOR THE JANUARY 2018 TRANSACTIONS BELOW FOR SUNFLOWER DESIGNS: a. On January 1, $350 of office supplies was purchased on credit; the terms of the invoice were net 30. All of...
-
The following is a list of the six largest pharmaceutical and biotech companies in the world ranked by health-care revenue (in US$ millions). Use this information to construct a pie chart and a bar...
-
Plot the shapes of the optical and acoustic branches in the dispersion relation for four different ratios of masses: and 1. Show that, in the case of two identical atoms, there is actually only one...
-
In Sect. 6.1.4, we calculated the ratio of the displacement amplitudes A and B for the long wave limit (k ! 0) for both the optical and acoustic phonon branches and then determined the displacement...
-
Indicate whether each of the following statements is true or false by writing T or F in t he a nswer c olumn. Title is proof of ownership as shown by a bill of sale.
-
Lennys Limousine Service (LLS) is considering the purchase of two Hummer limousines. Various information about the proposed investment follows: Required: Help LLS evaluate this project by calculating...
-
Lancer Corp. has the following information available about a potential capital investment Required: 1. Calculate the projects net present value. 2. Without making any calculations, determine whether...
-
Woodchuck Corp. is considering the possibility of outsourcing the production of upholstered chair pads included with some of its wooden chairs. The company has received a bid from Padalong Co. to...
-
Woodchuck Corp. is considering eliminating a product from its line of outdoor tables. Two products, the Oak-A and Fiesta tables, have impressive sales. However, sales for the Studio model have been...
-
Suppose that Flyaway Company also produces the Windy model fan, which currently has a net loss of \($40,000\) as follows: Eliminating the Windy product line would eliminate \($20,000\) of direct...
-
Peel Company owns 90% of the common stock of Seacore Company. Seacore Company sells merchandise to Peel Company at 20% above cost. During 2019 and 2020, such sales amounted to $436,000 and $532,000,...
-
A random sample of 10 houses heated with natural gas in a particular area, is selected, and the amount of gas (in therms) used during the month of January is determined for each house. The resulting...
-
Make a graph of [Ag + ], [AgOH(aq)], [CN - ], and [HCN] as a function of pH in a saturated solution of AgCN. Consider the following equilibria and do not consider activity coefficients. Find the pH...
-
Difference plot. A solution containing 3.96 mmol acetic acid plus 0.484 mmol HCI in 200 mL, of 0.10 M KC1 was titrated with 0.490 5 M NaOH to measure K. for acetic acid. (a) Write expressions for the...
-
Difference plot. A solution containing 3.96 mmol acetic acid plus 0.484 mmol HCI in 200 mL, of 0.10 M KC1 was titrated with 0.490 5 M NaOH to measure K. for acetic acid. (a) Write expressions for the...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...
-
The wash sale rules apply to disallow a loss on a sale of securities_______? Only when the taxpayer acquires substantially identical securities within 30 days before the sale Only when the taxpayer...

Study smarter with the SolutionInn App


