The figure below illustrates measurements of the specific heat (plotted as C/T versus T 2 ) for
Question:
The figure below illustrates measurements of the specific heat (plotted as C/T versus T2) for a crystalline element. Use what you know about the origins and temperature dependence of the specific heat capacity to determine whether the element is Na or Si. Discuss both possibilities.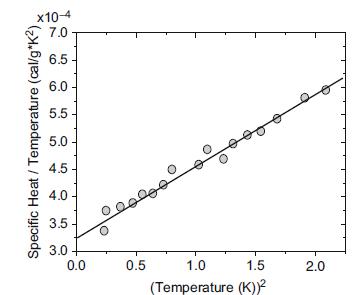 Experimental data of the specific heat of an unknown element.
Experimental data of the specific heat of an unknown element.
Transcribed Image Text:
x10-4 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 - 000000 3.0 0.0 0.5 1.0 1.5 2.0 (Temperature (K) Specific Heat / Temperature (cal/g*K?),
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Based on the provided data we can compare the specific heat capacity curve to the expected behavior ...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Use what you know about enzymes to propose an explanation for why our bodies cannot digest the cellulose in dietary fiber. Also, why would a cells fat-digesting enzymes not be able to digest an...
-
For each of the following, review what you know about the mission and business activities of Marathon Vitamin Shops. Then make a recommendation regarding each option the analysts and clients have...
-
The figure below illustrates the change in consumer surplus, given by Area ABEC, when the price decreases from P1 to P2. This area can be divided into the rectangle ABDC and the triangle BDE. Briefly...
-
Answer Problem 6.12 for a 90% CI. Refer to the data in Table 2.13. Regard this hospital as typical of Pennsylvania hospitals. Table 2.13: Hospital-stay data
-
Victoria is overseeing the accounts receivable ledger. Her main focus is the posting of entries for sales and sales returns to the control account and the individual accounts receivable accounts. The...
-
When a motor armature inertia, a pinion inertia, and a motor torque reside on a motor shaft, and a gear inertia, a load inertia, and a load torque exist on a second shaft, it is useful to reflect all...
-
2. Prepare journal entries to record collection of the receivable and settlement of the forward contract on February 28.
-
What are the ethical implications for leaders who ignore the impacts of severe workplace stress on their employees?
-
Big Deal Inc.: (Part 4 of 5) Use the information above for Big Deal Inc. to answer the following question: If Big Deal's cash flow statement was completed using the direct method, what is the sum of...
-
Answer the following questions related to the Docks Creek Land Company case presented in the chapter: 1. How does Robertsons role differ from Wisemans? 2. Are the professional standards applied...
-
From the figures for the phonon dispersion curves for Si and GaAs plus the equations for optical and acoustic phonons, explain why the energy for the Si curves is higher in energy than the curves for...
-
Plot the average number of phonons for at least five values of T to show its evolution with increasing temperatures. For each one, plot the function and show that it is a good approximation for N()...
-
Using the Internet and search engines, visit a website that provides a free download to detect spyware.
-
Classic Auto Parts sells new and used auto parts. Although a majority of its sales are cash sales, it makes a significant amount of credit sales. During 2012, its first year of operations, Classic...
-
The following information is available for Market Inc. and Supply Inc. at December 31, 2012: Required a. What is the accounts receivable turnover for each of the companies for 2012 ? b. What is the...
-
Buck Novak, the chief executive officer of Novak Corporation, has assembled his top advisers to evaluate an investment opportunity. The advisers expect the company to pay \($400,000\) cash at the...
-
Verify the log-likelihood in equation (16.4) for the Tobit model. In L = = In { 1-0 (x-di)} 1:y=di 122. + (y; - x) 02 (16.4) i:y;>di
-
Milo Company is considering the purchase of new equipment for its factory. It will cost \($250,000\) and have a \($50,000\) salvage value in five years. 1 he annual net income from the equipment is...
-
On January 1, 2019, Pearce Company purchased an 80% interest in the capital stock of Searl Company for $2,460,000. At that time, Searl Company had capital stock of $1,500,000 and retained earnings of...
-
If a test has high reliability. O the test measures what the authors of the test claim it measures O people who take the same test twice get approximately the same scores both times O scores on the...
-
Which will be the strongest oxidizing agent under standard conditions (that is, all activities = 1): HNO 2 , Se, UO 2 2+ , Cl 2 , H 2 SO 3 , or MnO 2 ?
-
What is the difference between E and E for a redox reaction? Which one runs down to 0 when the complete cell comes to equilibrium?
-
(a) Use the Nernst equation to write the spontaneous chemical reaction that occurs in the cell in Demonstration 13-1. (b) If you use your fingers as a salt bridge in Demonstration 13-1, will your...
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


