Determine the reactions of structure B -3m- .3m 4m D 20 kN 15 KN
Question:
Determine the reactions of structure
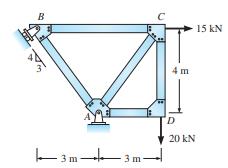
Transcribed Image Text:
B -3m- .3m 4m D 20 kN 15 KN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
nt 0 0 0 R5 15 4 20 30 111 R24 kN R ...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Structural Analysis
ISBN: 9780073398006
5th Edition
Authors: Kenneth Leet, Chia-Ming Uang, Joel Lanning
Question Posted:
Students also viewed these Engineering questions
-
Problem 2. Based on a previous midterm exam problem. This problem asks you to analyze the effect of using a "bumpless" feedback control implementation (Figure 1). C2 + A- C3 Figure 1: "Bumpless"...
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
With current spectroscopic techniques (discussed in Chapters 1517), chemists are generally able to determine the structure of an unknown organic compound in just one day. These techniques have only...
-
Show that the rectangular box (including the top and bottom) with fixed volume V = 27 m 3 and smallest possible surface area is a cube (Figure 25). X y Z
-
A fixed force center scatters a particle of mass m according to the force law F(r) = k/r3. If the initial velocity of the particle is u0, show that the differential scattering cross section is km (T...
-
Some scholars have argued that the same standardized marketing strategy should be adopted for all foreign markets. Does this imply that the marketing research process should also be standardized and...
-
P3-10 Calculate investment cost and account balances from a consolidated balance sheet five years after acquisition The consolidated balance sheet of Pam Corporation and its 80 percent subsidiary,...
-
Garrison holds a controlling interest in Robertsons outstanding stock. For the current year, the following information has been gathered about these two companies: Garrison uses the cost method to...
-
In 2015, a baseball player signed a contract reported to be worth $101.4 million. The contract was to be paid as $15.2 million in 2015, $15.3 million in 2016, $17.6 million in 2017, $17.7 million in...
-
Determine the reactions of structure 1.2 kips/ft B A 18k 12'- D 9
-
Determine the reactions of structure 15 kip. ft 5 5' B D 12] E 8 kips 6 kips 10'
-
What is the maximum value of the step response as a function of \(\zeta\) ?
-
Carol's Cupcakes has grown from a home business into a one of the largest event and wedding catering companies in the area. Its founder, Carol Thompson, first dreamed of owning her own company while...
-
Many things have changed for businesses in 2022. The previous 2 business years of 2020 and 2021 have tested businesses and the workforce like nothing else. Not only were profits reduced, and...
-
1) Virginia Tech's motto is "Ut Prosim" which means 'That I May Serve'. Share how you contribute to a community that is important to you. How long have you been involved? What have you learned and...
-
Person Is Arianna Grande Answer all questions Who are they? How successful are they? Why would companies be interested in partnering with them? Identify one company from their industry that you feel...
-
Imagine you have just retired after a long and very successful career (as a physiotherapist). Congratulations! You've made such an impact in the world that business and community leaders from around...
-
Air enters an evaporative cooler at 1 atm, 32oC, and 30 percent relative humidity at a rate of 5m3/min and leaves at 22oC. Determine (a) The final relative humidity. (b) The amount of water added to...
-
d) For die casting processes: 1. What are the most common metals processed using die casting and discuss why other metals are not commonly die casted? 2. Which die casting machines usually have a...
-
Using activities, find the concentrations of the major species in 0.10 M NaClO 4 saturated with Mn(OH) 2 . Take the ionic strength to be 0.10 M and suppose that the ion size of MnOH + is the same as...
-
Explain why the solubility of an ionic compound increases as the ionic strength of the solution increases (at least up to ~ 0.5 M).
-
Which statements are true? In the ionic strength range 00.1 M, activity coefficients decrease with (a) Increasing ionic strength; (b) Increasing ionic charge; (c) Decreasing hydrated radius.
-
Present Value Computations Using the present value tables, solve the following. ( Click here to access the PV and FV tables to use with this problem. ) Round your answers to two decimal places....
-
A company provided the following data: Sales $887,000 Variable costs $546,800 Fixed costs $310,000 Expected production and sales in units 36,000 What is the break-even point in sales dollars? Please...
-
How to solve them..equation and explain ..please.. 1. Selected information from the companys financial records is presented below Equipment, December 31, 2013 $300,000 Equipment, December 31, 2014...

Study smarter with the SolutionInn App


