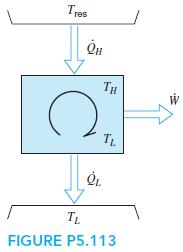
A Carnot heat engine, shown in Fig. P5.113, receives energy from a reservoir at Tres through a
Question:
A Carnot heat engine, shown in Fig. P5.113, receives energy from a reservoir at Tres through a heat exchanger where the heat transferred is proportional to the temperature difference as ˙Q H = K(Tres − TH). It rejects heat at a given low temperature TL. To design the heat engine for maximum work output, show that the high temperature, TH, in the cycle should be selected as TH =√TresTL
Transcribed Image Text:
Tres TH TL TL FIGURE P5.113
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
TH TresTL The maximum work output of the Carnot heat engine occurs when the heat exchange is maximiz...View the full answer

Answered By

Firoz K
I have extensive experience in education and tutoring, having worked as a tutor for the past three years in both group and individual settings. During my time as a tutor, I have successfully helped students improve their academic performance in a variety of subjects, including mathematics, science, language arts, and social studies. I have also developed and implemented personalized learning plans and differentiated instruction techniques to accommodate the individual needs of my students. Moreover, I have effectively communicated with parents and teachers to ensure that the students receive the best possible education and guidance. My strong organizational, communication, and problem-solving skills have enabled me to successfully collaborate with students, parents, and teachers in order to provide an effective and enjoyable learning experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A Carnot heat engine receives energy from a reservoir at Tres through a heat exchanger where the heat transferred is proportional to the temperature difference as QH = K (Tres - TH). It rejects heat...
-
A Carnot heat engine receives heat from a reservoir at 1700F at a rate of 700 Btu/min and rejects the waste heat to the ambient air at 80F. The entire work output of the heat engine is used to drive...
-
A Carnot heat engine is operating between a source at TH and a sink at TL. If it is desired to double the thermal efficiency of this engine, what should the new source temperature be? Assume the sink...
-
Suppose you want to buy a house that is sold by way of a first-price sealed bid auction. In contrast to the model in the lecture, there are more than 2 players. Players simultaneously and...
-
You are interested in studying the variability of crimes committed (including violent and property crimes) and police expenditures in the eastern and Midwestern United States. The U.S. Census Bureau...
-
Two 20-in. rods AB and DE are connected as shown. Point D is the midpoint of rod AB, and at the instant shown rod DE is horizontal. Knowing that the velocity of point A is 1 ft/s downward, determine...
-
Are stocks and bonds complements? Explain.
-
The following events occur for Morris Engineering during 2015 and 2016, its first two years of operations. February 2, 2015 Provide services to customers on account for $ 38,000. July 23, 2015...
-
Using FIFO, what is gross margin? Purchase $ per Date Activity Units unit Total cost Beginning 875 $48 $42,000 1-Jan balance 10-Jan Purchased 630 $46 $28,980 13-Feb Purchased 940 $52 $48,880 Cost of...
-
Star Videos, Inc., produces short musical videos for sale to retail outlets. The company's balance sheet accounts as of January 1 are given below. Because the videos differ in length and in...
-
Air in a rigid 1-m 3 box is at 300 K, 200 kPa. It is heated to 600 K by heat transfer from a reversible heat pump that receives energy from the ambient at 300 K besides the work input. Use constant...
-
A combination of a heat engine driving a heat pump (see Fig. P5.114) takes waste energy at 50C as a source Q w1 to the heat engine, rejecting heat at 30C. The remainder, Q w2 , goes into the heat...
-
Reed owned a small photography store and purchased his price label products from Monarch. Over a period of years, Reed ordered no more than 4,000 labels at a time from Monarch. While preparing a new...
-
How do emergent properties within teams, such as synergy and collective intelligence, manifest and influence team performance, and what factors contribute to their development and sustenance?
-
Think about your workplace, organization, or industry; if you are not currently working, think about previous employment or a job that you are aiming for. The broader your perspective, the more...
-
Choose any global organization that successfully undertook a strategic transformation to adapt to changing market dynamics and sustain its competitive advantage. Examine the company's challenges,...
-
In examining C&C Sports through the lens of a SWOT analysis, several key factors come to light. The strengths of the company are evident in its established brand reputation and a loyal customer base...
-
A perfectly insulated container initially contains 0.2 kg of ice at -15 C. Now we add water at 30 C, but only the minimum amount needed to barely melt all the ice. Find the net entropy change of the...
-
ISBN-13 is a new standard for indentifying books. It uses 13 digits d 1 d 2 d 3 d 4 d 5 d 6 d 7 d 8 d 9 d 10 d 11 d 12 d 13 . The last digit d 13 is a checksum, which is calculated from the other...
-
Classify each of the following activities as proper or prohibited under the various consumer statutes you have studied. a. Calling a hospital room to talk to a debtor who is a patient there. b....
-
Aspartame (below) is an artificial sweetener used in diet soft drinks and is marketed under many trade names, including Equal TM and Nutrasweet TM . In the body, aspartame is hydrolyzed to produce...
-
Draw a plausible mechanism for each of the following transformations: a. b. c. d. e. Pyridine CI
-
Ethyl trichloroacetate is significantly more reactive toward hydrolysis than ethyl acetate. Explain this observation.
-
If the auditor believes that the financial statements prepared on the basis of the entity's income tax are not adequately titled, the auditor should : A)Issue a resignation of opinion. B)Explain the...
-
initial stock offering to the public. This REIT specializes in the acquisition and management of warehouses. Your firm, Blue Street Advisors, is an investment management company that is considering...
-
Question 3 You have been hired to run a pension fund for Mackay Inc, a small manufacturing firm. The firm currently has Gh5 million in the fund and expects to have cash inflows of $2 million a year...

Study smarter with the SolutionInn App


