A coflowing (same direction) heat exchanger, shown in Fig. P7.113, has one line with 0.5 kg/s oxygen
Question:
A coflowing (same direction) heat exchanger, shown in Fig. P7.113, has one line with 0.5 kg/s oxygen at 17◦C, 200 kPa entering, and the other line has 0.6 kg/s nitrogen at 150 kPa, 500 K entering. The heat exchanger is very long, so the two flows exit at the same temperature. Use constant heat capacities and find the exit temperature and the total rate of entropy generation.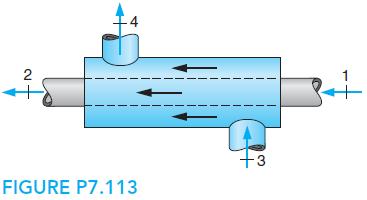
Transcribed Image Text:
4 2 +3 FIGURE P7.113
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
To find the exit temperature of the heat exchanger we can use the energy balance equation for both the oxygen and nitrogen streams Since the heat exch...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A condenser, as the heat exchanger shown in Fig P6.84 brings 1 lbm/s water flow at 1 lbf/in.2 from 500 F to saturated liquid at 1 lbf/in.2. The cooling is done by lake water at 70 F that returns to...
-
A condenser, as the heat exchanger shown in Fig P6.14, brings 1 lbm/s water flow at 1 lbf/in 2 from 500 F to saturated liquid at 1 lbf/in 2. The cooling is done by lake water at 70 F that returns to...
-
The dynamic behavior of the heat exchanger shown in figure can be described by the following transfer functions The valve lift x is measured in inches. Other symbols are defined in Figure.(a) Find...
-
Joseph Kent started business on 1 July 2018 as a joiner making conservatories. His taxadjusted profits (before deduction of capital allowances) were as follows: Private use of both cars has been...
-
A numerical control drill press drills four 10.0 mm diameter holes at four locations on a flat aluminum plate in a production work cycle. Although the plate is only 12 mm thick, the drill must travel...
-
Evaluate the indefinite integral. Illustrate and check that your answer is reasonable by graphing both the function and its anti-derivative (take C = 0). 52. Vr + 1 51. ( In(x? + 2.x + 2) dx dx
-
How do you use IQR to check that the data came from a standard normal distribution? LO8
-
Suppose Levered Bank is funded with 2% equity and 98% debt. Its current market capitalization is $10 billion, and its market to book ratio is 1. Levered Bank earns a 4.22% expected return on its...
-
Exercise 6-9 Bank reconciliation and adjusting entries LO P3 A table for a monthly bank reconciliation dated September 30 is given below. For each item 1 through 12, indicate whether the item should...
-
For this assessment, you will choose a local or global issue that pertains to technology. The chosen issue should also relate to one or more of the topics that have been addressed in this course...
-
A 1-m 3 rigid tank contains 100 kg R-410a at a temperature of 15C, as shown in Fig. P7.116. A valve on top of the tank is opened, and saturated vapor is throttled to ambient pressure, 100 kPa and...
-
One type of feedwater heater for preheating the water before entering a boiler operates on the principle of mixing the water with steam that has been bled from the turbine. For the states shown in...
-
The net income (after income tax) of Choi Inc. was $15 per common share in the latest year and $60 per common share for the preceding year. At the beginning of the latest year, the number of shares...
-
Evaluation a. Evaluate the effectiveness of social media marketing campaign for instagram, facebook and pintrest ?based on your KPIs for example account reached, content reached, likes, shares,...
-
A study was performed at a university to analyze whether the preference for hamburgers or fried chicken is related to the gender of the student. This table lists the results of the study. At a =...
-
A 20-lb homogeneous box has tipped and is resting against a 40-lb homogeneous box as shown in figure attached. The coefficient of friction between box A and the floor is 0.7, and between box B and...
-
The Taylor series for natural logarithm (with base e) In(1+r) is In(1+2) -(-1)+1 for <1. (a) Write a user-defined function using loop that determines In(1+x) using the above Taylor series. Your...
-
Question 1: [up to 4 pts] Suppose that a = 1, a2 = 2, a3 = = 3, and an = an-3 for all n 4. If an integral with respect to y is used to find the area of R, what should the upper limit of integration...
-
On May 31, 2022, Reber Company had a cash balance per books of $6,781.50. The bank statement from New York State Bank on that date showed a balance of $6,404.60. A comparison of the statement with...
-
You purchase a bond with a coupon rate of 6.7 percent, a par value $1,000, and a clean price of $905. Assume a par value of $1,000. If the next semiannual coupon payment is due in two months, what is...
-
Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
-
Liquid N 2 has a density of 875.4 kg m 3 at its normal boiling point. What volume does a balloon occupy at 298 K and a pressure of 1.00 atm if 3.10 10 3 L of liquid N 2 is injected into it? Assume...
-
Calculate the volume of all gases evolved by the complete oxidation of 0.375 g of the amino acid alanine NH 2 CHCH 3 COOH if the products are liquid water, nitrogen gas, and carbon dioxide gas, the...
-
Trey is single and has no qualifying child. His adjusted gross income is $12,355. In order to claim the Earned Income Tax Credit, he must meet which of the following requirements? He cannot be the...
-
Caspian Sea Drinks needs to raise $74.00 million by issuing additional shares of stock. If the market estimates CSD will pay a dividend of $2.69 next year, which will grow at 3.45% forever and the...
-
i need help in B and C Integrative Case 5-72 (Algo) Cost Estimation, CVP Analysis, and Decision Making (LO 5-4.5.9) Luke Corporation produces a variety of products, each within their own division....

Study smarter with the SolutionInn App


