A piston/cylinder contains 0.5 kg of water at 200 kPa, 300C, and it now cools to 150C
Question:
A piston/cylinder contains 0.5 kg of water at 200 kPa, 300◦C, and it now cools to 150◦C in an isobaric process. The heat goes into a heat engine that rejects heat to the ambient at 25◦C (shown in Fig. P6.46), and the whole process is assumed to be reversible. Find the heat transfer out of the water and the work given out by the heat engine.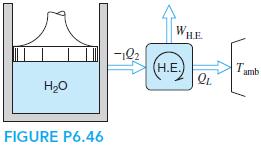
Transcribed Image Text:
WHE -12 (Н.Е. T. amb H20 FIGURE P6.46
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Q mCT3T2 W mpCT3T1 The heat lost by the water is calculated as follows Q 0510300 ...View the full answer

Answered By

Enock Oduor
I am a chemist by profession, i coach high school students with their homework, i also do more research during my free time, i attend educational and science fair seminars where i meet students and do some projects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A piston/cylinder contains 50 kg of water at 200 kPa with a volume of 0.1 m3. Stops in the cylinder are placed to restrict the enclosed volume to 0.5 m3 similar to Fig. P5.20, the water is now heated...
-
A piston/cylinder contains 50 kg of water at 200 kPa with a volume of 0.1 m3. Stops in the cylinder restricts the enclosed volume to 0.5 m3, similar to the setup in Problem 4.7. The water is now...
-
A piston/cylinder contains 50 kg of water at 200 kPa with a volume of 0.1 m3. Stops in the cylinder are placed to restrict the enclosed volume to a maximum of 0.5 m3. The water is now heated until...
-
Nancy takes out a 29-year loan of $650,000 today. The bank charges interest at 3.6% p.a. compounded monthly. Nancy makes equal month-end payments starting in one month's time. Calculate how much...
-
Suppose you are using a sample from the 2010 GSS data for a research project on education in the United States. The GSS includes a question that asks for the number of years of education. Based on...
-
A 5000-lb probe in orbit about the moon is 8 ft high and has octagonal bases of sides 4 ft. The coordinate axes shown are the principal centroidal axes of inertia of the probe, and its radii of...
-
Think of a small work group that you belonged to recently. Assess the level of its cohesiveness. What factors contributed to, or prevented, its cohesiveness? lop5
-
Assume a federal agency has the following events: 1. Receives a warrant from the Treasury notifying the agency of appropriations of $2,400,000. 2. OMB apportions one-fourth of the appropriation for...
-
Carmen Camry operates a consulting firm called Help Today, which began operations on August 1. On August 31, the companys records show the following selected accounts and amounts for the month of...
-
Josephine Mater works for the supply-chain analytics division of Trader Joes, a national chain of specialty grocery stores. Trader Joes is considering a redesign of its supply chain. Josephine knows...
-
A piston/cylinder contains 0.25 kg of R-134a at 100 kPa. It will be compressed in an adiabatic reversible process to 400 kPa and should be at 70C. What should the initial temperature be?
-
Water in a piston/cylinder at 400C, 2000 kPa is expanded in a reversible adiabatic process. The specific work is measured to be 415.72 kJ/kg out. Find the final P and T and show the Pv and Ts...
-
A rock bed consists of 6000 kg granite and is at 70C. A small house with lumped mass of 12000 kg wood and 1000 kg iron is at 15C. They are now brought to a uniform final temperature with no external...
-
Systems thinking is all about solving problemsin organizations, world situations, and even our personal lives. But it is not just a procedure; it is a different way of approaching problems. Our...
-
How would I display the following 3 principles in an entertaining infographic? Be very specific . Principle 1: Employee Engagement and Motivation Drawing from the Human Relations Movement theory and...
-
Shown below is a cross section of tubular member which is subjected to a torque T= 5.5 kN-m. It has a length L-3.0-m and the material shear modulus G=27 GPa. Dimensions: b=150 mm, h= 100 mm and t= 8...
-
The hip roof shown in the below Figure 2 is constructed of 2x10 rafters spaced 16 inches on center. The hip rafters are 1 -inch-wide by 12-inch-high GLBs. The roof has a slope of 4:12. Prepare a list...
-
2. Estimate the populations of Fargo, ND and Bismarck, ND in years of 2040 and 2050. Select a single value of population that you would use for design purposes in each year. You need to specify and...
-
In late July 2020, Mona Ltd., a private company, paid $2 million to acquire all of the net assets of Lubello Corp., which then became a division of Mona. Lubello reported the following statement of...
-
What is an access control list?
-
Dichloroacetic acid has a dissociation constant of K a = 3.32 10 -2 . Calculate the degree of dissociation for a 0.105 m solution of this acid a. Using the DebyeHckel limiting law using an iterative...
-
Calculate the DebyeHckel screening length 1/ at 298 K in a 0.0075 m solution of K 3 PO 4 .
-
Calculate I, , and a for a 0.0215 m solution of K 2 SO 4 at 298 K. How confident are you that your calculated results will agree with experimental results?
-
Production numbers for 2 shifts are shown. The shift supervisor of Shift 2 insists to the production manager that her operators are more productive than the ones on Shift 1. Using a confidence level...
-
In a class, the scores that students got are as shown. What are the 25, 50, 75 and 100th percentiles for the data? 84 84 98 80 89 83 85 56 85 84 84 74 84 81 83 80 45 86 67 79 81 78 76 85 83 77 86 83...
-
Number of points made by Teams A and B are shown. Which statement is true based on running the F-Test Two-Sample for Variances in the Data Analysis pack in Excel? Use a confidence level of 10% to...

Study smarter with the SolutionInn App


