A proposal is to build a 1000-MW electric power plant with steam as the working fluid. The
Question:
A proposal is to build a 1000-MW electric power plant with steam as the working fluid. The condensers are to be cooled with river water (see Fig. P5.61). The maximum steam temperature is 550◦C, and the pressure in the condensers will be 10 kPa. Estimate the temperature rise of the river downstream from the power plant.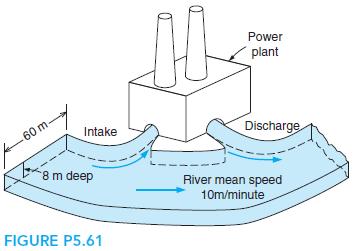
Transcribed Image Text:
Power plant 60 m. Intake Discharge B8m deep River mean speed 10m/minute FIGURE P5.61
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A commercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at -35°C by rejecting waste heat to cooling water that enters the condenser at 18°C...
-
A heat pump with refrigerant-134a as the working fluid is used to keep a space at 25°C by absorbing heat from geothermal water that enters the evaporator at 60°C at a rate of 0.065 kg/s and...
-
A glass manometer with oil as the working fluid is connected to an air duct as shown in Fig. P12??11. Will the oil levels in the manometer be as in Fig. P12??11Ca or b? Explain. What would your...
-
Relationship of the Balanced Scorecard to activity-based costing Explain how an activity-based costing model can be linked to a Balanced Scorecard approach.
-
a. For each situation in Exercise 3, describe the Type I and Type II errors that could occur. b. What are the general implications of making a Type I error? Of making a Type II error? c. When would...
-
The triple jump is a track-and-field event in which an athlete gets a running start and tries to leap as far as he can with a hop, step, and jump. Shown in the figure is the initial hop of the...
-
In their text, Q~~antitative Analysis of Management (1997), B. Render (Rollins College) and R. M. Stair (Florida State University), present the case of the Bayfield Mud Company. Bayfield supplies...
-
1. Develop a graph or table that portrays the checking balances. What is the balance of a typical customer? Do many customers have more than $2,000 in their ac-counts? Does it appear that there is a...
-
Remaining Time: 44 minutes, 38 seconds. Question Completion Status: 1 2 3 5 6 7 8 9 10 11 Problem 1: Answer the following question and show all calculations (5 Marks): Presented below is information...
-
In a survey of 130 people who used food delivery services, it was determined that 74 used Grubhub. 70 used Uber Eats. 41 used both Grubhub and Uber Eats. Of those surveyed, a) How many used only...
-
A heat pump is used to heat a house during the winter. The house is to be maintained at 20C at all times. When the ambient temperature outside drops to 10C, the rate at which heat is lost from the...
-
A constant temperature of 125C must be maintained in a cryogenic experiment, although it gains 120 W due to heat transfer. What is the smallest motor you would need for a heat pump absorbing heat...
-
Determine the one or two steps it takes to get from the starting material to the product using the reactions found in this chapter. a. HC HC OH OH c. CH3CH,C=CH, HC C CH-OH T b. CH3 -CH-CH-CH-CH3 T...
-
Read the buret (burette) volume and report your reading with the proper number of digits. Number 3.2 mL mL 0 10 15 46 20 25 30 35 47 40 48 Incorrect.
-
TCP Congestion Control using Wireshark and testmy.net. Identify the IP Address, Protocol (UDP or TCP), Destination and Source IP Address, and IP Class Type (A-D).
-
Charley & Waldo's World of Wonder is a science-oriented children's museum. The museum has a "free" section where children have unlimited use science oriented exhibits and a premium section where...
-
Let f(x) In(x). Solve each of the following equations exactly for a. (f(x)) = 11 b. f(x) = 11 c. f(x) = 11
-
Suppose the annual rate of inflation in Taiwan is 6.66%, and the annual rate of inflation in Mexico is 5.99%. If the Mexican peso depreciates relative to the Taiwan dollar by 4% in real terms, then...
-
Write a method for solving a quadratic equation using the following header: public static int solveQuadratic(double[] eqn, double[] roots) The coefficients of a quadratic equation ax 2 + bx + c = 0...
-
On August 31, 2012, the balances of the accounts appearing in the ledger of Wood Interiors Company, a furniture wholesaler, are as follows:Prepare the August 31, 2012, closing entries for Wood...
-
An ideal gas undergoes an adiabatic expansion into a vacuum. Are S, S surroundings , and S total positive, negative, or zero? Explain your reasoning.
-
Propose a plausible synthesis for each of the following transformations. a. b. c. d. H.
-
When a saturated solution of a salt is cooled, a precipitate crystallizes out. Is the entropy of the crystalline precipitate greater or less than the dissolved solute? Explain why this process is...
-
Current Portion of Long-Term Debt PepsiCo, Inc., reported the following information about its long-term debt in the notes to a recent financial statement (in millions): Long-term debt is composed of...
-
Show transcribed image text 31/12/2016 GHS'000 25,500 The following information relates to the draft financial statements of Samanpa Ltd. Summarised statement of financial position as at: 31/12/2017...
-
\ How do i solve this? Beginning raw materials inventory Ending raw materials inventory Direct labor Operating expenses Purchases of direct materials Beginning work in process inventory Ending work...

Study smarter with the SolutionInn App


