An air pistol contains compressed air in a small cylinder, as shown in Fig. P3.164. Assume that
Question:
An air pistol contains compressed air in a small cylinder, as shown in Fig. P3.164. Assume that the volume is 1 cm3, the pressure is 1MPa, and the temperature is 27◦C when armed. A bullet, with m = 15 g, acts as a piston initially held by a pin (trigger); when released, the air expands in an isothermal process (T = constant). If the air pressure is 0.1 MPa in the cylinder as the bullet leaves the gun, find
a. The final volume and the mass of air
b. The work done by the air and the work done on the atmosphere
c. The work done to the bullet and the bullet exit velocity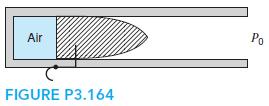
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:





