Natural gas, we assume methane, is burned with 200% theoretical air, shown in Fig. P13.75, and the
Question:
Natural gas, we assume methane, is burned with 200% theoretical air, shown in Fig. P13.75, and the reactants are supplied as gases at the reference temperature and pressure. The products are flowing through a heat exchanger, where they give off energy to some water flowing in at 20◦C, 500 kPa, and out at 700◦C, 500 kPa. The products exit at 400 K to the chimney. How much energy per k mole fuel can the products deliver, and how many kilograms of water per kilogram of fuel can they heat?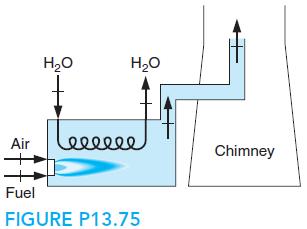
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:





