Gasoline, C 7 H 17 , is burned in a steady-state burner with stoichiometric air at P
Question:
Gasoline, C7H17, is burned in a steady-state burner with stoichiometric air at P0, T0, shown in Fig. P13.77. The gasoline is flowing as a liquid at T0 to a carburetor, where it is mixed with air to produce a fuel air–gas mixture at T0. The carburetor takes some heat transfer from the hot products to do the heating. After the combustion, the products go through a heat exchanger, which they leave at 600 K. The gasoline consumption is 10 kg/h. How much power is given out in the heat exchanger, and how much power does the carburetor
need?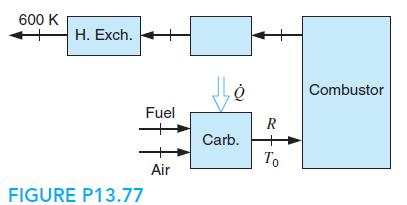
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:





