We want to find the change in u for carbon dioxide between 50C and 200C at a
Question:
We want to find the change in u for carbon dioxide between 50◦C and 200◦C at a pressure of 10 MPa. Find it using ideal gas and Table A.5, and repeat using the B section table.
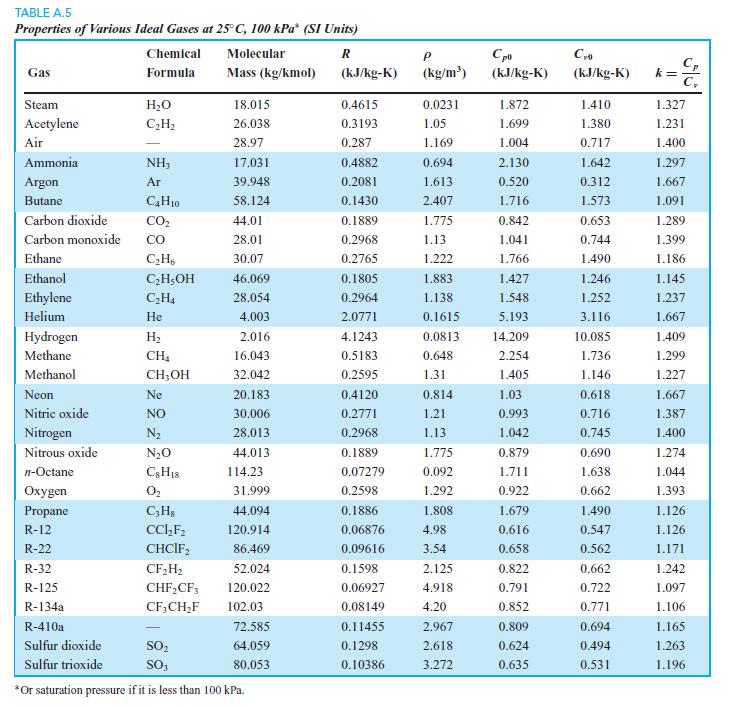
Transcribed Image Text:
TABLE A.5 Properties of Various Ideal Gases at 25° C, 100 kPa* (SI Units) Chemical Molecular C, k = C, Gas Formula Mass (kg/kmol) (kJ/kg-K) (kg/m') (kJ/kg-K) (kJ/kg-K) Steam H2O 18.015 0.4615 0.0231 1.872 1.410 1.327 Acetylene C;H2 26.038 0.3193 1.05 1.699 1.380 1.231 Air 28.97 0.287 1.169 1.004 0.717 1.400 Ammonia NH3 17.031 0.4882 0.694 2.130 1.642 1.297 Argon Ar 39.948 0.2081 1.613 0.520 0.312 1.667 Butane C,H10 58.124 0.1430 2.407 1.716 1.573 1.091 Carbon dioxide CO, 44.01 0.1889 1.775 0.842 0.653 1.289 Carbon monoxide со 28.01 0.2968 1.13 1.041 0.744 1.399 Ethane C;H, 30.07 0.2765 1.222 1.766 1.490 1.186 Ethanol C,H;OH 46.069 0.1805 1.883 1.427 1.246 1.145 Ethylene C,H4 28.054 0.2964 1.138 1.548 1.252 1.237 Helium Не 4.003 2.0771 0.1615 5.193 3.116 1.667 Hydrogen H2 2.016 4.1243 0.0813 14.209 10.085 1.409 Methane CH4 16.043 0.5183 0.648 2.254 1.736 1.299 Methanol CH,OH 32.042 0.2595 1.31 1.405 1.146 1.227 Neon Ne 20.183 0.4120 0.814 1.03 0.618 1.667 Nitric oxide NO 30.006 0.2771 1.21 0.993 0.716 1.387 Nitrogen N2 28.013 0.2968 1.13 1.042 0.745 1.400 Nitrous oxide N20 44.013 0.1889 1.775 0.879 0.690 1.274 n-Octane 114.23 0.07279 0.092 1.711 1.638 1.044 Охудen O2 31.999 0.2598 1.292 0.922 0.662 1.393 Propane C;H3 44.094 0.1886 1.808 1.679 1.490 1.126 R-12 CC,F; 120.914 0.06876 4.98 0.616 0.547 1.126 R-22 CHCIF, 86.469 0.09616 3.54 0.658 0.562 1.171 R-32 CF,H; 52.024 0.1598 2.125 0.822 0.662 1.242 R-125 CHF,CF; 120.022 0.06927 4.918 0.791 0.722 1.097 R-134a CF,CH,F 102.03 0.08149 4.20 0.852 0.771 1.106 R-410a 72.585 0.11455 2.967 0.809 0.694 1.165 Sulfur dioxide SO, 64.059 0.1298 2.618 0.624 0.494 1.263 Sulfur trioxide SO, 80.053 0.10386 3.272 0.635 0.531 1.196 *Or saturation pressure if it is less than 100 kPa.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
u C t From table A5 C 0635 KJ Kg 1 K 1 Aga...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
We want to find the change in u for oxygen gas between 600 K and 1200 K. a) Find it from a constant Cvo from table A.5 b) Find it from a Cvo evaluated from equation in A.6 at the average T. c) Find...
-
We want to find the change in u for oxygen gas between 600 K and 1200 K. a) Find it from a constant Cvo from table A.5 b) Find it from a Cvo evaluated from equation in A.6 at the average T. c) Find...
-
In the following problems we want to find the Fourier transform of the signals. (a) For the signal find its Fourier transform by using the Fourier transform of x(t) = 0.5e at u(t) 0.5e at u(t), a >...
-
A 0.1 cm thick flat copper plate, 2.5 m x 2.5 m square is to be cooled in a vertical position. The initial temperature of the plate is 90?C with the ambient fluid at 30?C. The fluid medium is either...
-
Use the GSS10SSDS file to investigate whether or not Americans have at least two children per person. Use the One Sample T Test procedure to do this test with the variable CHILDS. Do the test at the...
-
A camera lens has a focal length of 180.0 mm and an aperture diameter of 16.36 mm. (a) What is the I-number of the lens? (b) If the correct exposure of a certain scene is 1/30S at f/11, what is the...
-
The EE curve is a representation of the foreign money market for a currency. Explain the similarities to the LM curve.
-
Use Appendix B.5 to locate the value of t under the following conditions. a. The sample size is 15 and the level of confidence is 95%. b. The sample size is 24 and the level of confidence is 98%. c....
-
I got the first 3 down I only have trouble with G H and I Thanks 18% Help s finance term case 2 (page 5 of 6) MINI CASE Andria Mullins, financial manager of Webster Electronics, has been asked by the...
-
Youve just been hired as an accountant at Murdstone, Inc., a retailer of supplies for arts and crafts. Since the previous accountant left his position suddenly, the controller of Murdstone, Inc. has...
-
Estimate the constant specific heats for R-134a from Table B.5.2 at 100 kPa and 125C. Compare this to the specific heats in Table A.5 and explain the difference.
-
For a special application, we need to evaluate the change in enthalpy for carbon dioxide from 30C to 1500C at 100 kPa. Do this using the constant specific heat value from Table A.5 and repeat using...
-
List the steps in a sampling plan for performing substantive tests of balances and transactions.
-
Alec is an employee who drives a 2021 Ford C-Max with a fair-market value of $32,000. He has been given the choice to have the fringe benefit reported on his W-2 either using the lease-value rule or...
-
What resource do most thinking and learning technologies rely on to be effective? a) data b) images c) gas d) robots
-
Financial Reporting Problem Marks and Spencer plc (M&S) The financial statements of M&S (GBR) are presented in Appendix A. The companys complete annual report, including the notes to the...
-
Totally Chemical is considering an investment decision project in which the organization expands into the trucking business. Totally Chemical wants to begin this investment decision project by buying...
-
Presented below is the balance sheet of Sandhill Corporation as of December 31, 2017. SANDHILL CORPORATION BALANCE SHEET DECEMBER 31, 2017 Goodwill (Note 2) Buildings (Note 1) Inventory Land Accounts...
-
Programming Exercise 5.45 computes the standard deviation of numbers. This exercise uses a different but equivalent formula to compute the standard deviation of n numbers. To compute the standard...
-
You have just begun your summer internship at Omni Instruments. The company supplies sterilized surgical instruments for physicians. To expand sales, Omni is considering paying a commission to its...
-
At the transition temperature of 95.4C, the enthalpy of transition from rhombic to monoclinic sulfur is 0.38 kJ mol 1 . a. Calculate the entropy of transition under these conditions. b. At its...
-
One mole of a van der Waals gas at 25.0????C is expanded isothermally and reversibly from an initial volume of 0.010 m 3 to a final volume of 0.095 m 3 . For the van der Waals gas, (U/V)T = a/V 2 m ....
-
From the following data, derive the absolute entropy of crystalline glycine at T = 300.K. You can perform the integration numerically using either a spreadsheet program or a curve-fitting routine and...
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...
-
On consolidated financial statements, where does the parents equity in the net income of the subsidiary account appear? A. On the consolidated income statement, as a revenue B. On the consolidated...

Study smarter with the SolutionInn App


