A 10.0 g sample of liquid water is sealed in a 1515 mL flask and allowed to
Question:
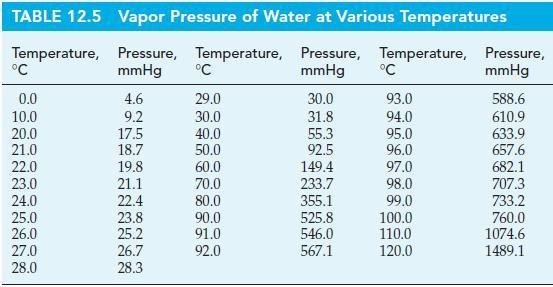
A 10.0 g sample of liquid water is sealed in a 1515 mL flask and allowed to come to equilibrium with its vapor at 27 °C. What is the mass of H2O(g) present when equilibrium is established? Use vapor pressure data from Table 12.5.
Table 12.5
Transcribed Image Text:
TABLE 12.5 Temperature, °C 0.0 10.0 20.0 21.0 22.0 23.0 24.0 25.0 26.0 27.0 28.0 Vapor Pressure of Water at Various Temperatures Pressure, Temperature, Pressure, Temperature, mmHg mmHg 4.6 9.2 17.5 18.7 19.8 21.1 22.4 23.8 25.2 26.7 28.3 °C 29.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 91.0 92.0 30.0 31.8 55.3 92.5 149.4 233.7 355.1 525.8 546.0 567.1 °C 93.0 94.0 95.0 96.0 97.0 98.0 99.0 100.0 110.0 120.0 Pressure, mmHg 588.6 610.9 633.9 657.6 682.1 707.3 733.2 760.0 1074.6 1489.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Find the vapor pressure of water at 27 C From Table 125 the vapor press...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
What will be the profit percentage after selling an article at certain price if there is a loss of 40 percent when the same article is sold at 2/5 of the earlier selling price?
-
Find given En+1 10 = 3 n = and = 4.
-
Which of the following are the government inspectors whose mission is to reduce Medicare improper payments through the detection and collection of overpayments, identification of underpayments, and...
-
In 1976, Mohamed EI-Iladad earned an undergraduate accounting degree in his native Egypt. Before he began his accounting career, El-Hadad completed his compulsory service in the Egyptian military...
-
Plaintiff brings this cause of action against a manufacturer for the loss of one leg below the hip. The leg was lost when caught in the gears of a screw auger machine sold and installed by the...
-
All of the following information would be included in the financial section of a CAFR except the a. letter of transmittal. b. Management's Discussion and Analysis. c. independent auditor's report. d....
-
Explain the similarities and differences between heritage attractions and commercial attractions.
-
Accounting for bonds using amortized cost measurement based on the historical market interest rate. Robinson Company issues $5,000,000 face value, 8% semiannual coupon bonds maturing in 10 years. The...
-
A firm with a WACC of 10% is considering the following mutually exclusive projects: 0 1 2 3 4 5 Project 1 -$400 $50 $50 $50 $225 $225 Project 2 -$450 $250 $250 $130 $130 $130 Which project would you...
-
A firm must decide between constructing a new facility or renting a comparable office space. There are two random outcomes for acquiring space, as shown in Figure PI 2-25. Each would accommodate the...
-
Some vapor pressure data for Freon-12, CCl 2 F 2 , once a common refrigerant, are -12.2 C, 2.0 atm; 16.1 C, 5.0 atm; 42.4 C, 10.0 atm; 74.0 C, 20.0 atm. Also, bp = -29.8 C, T c = 111.5 C, P c = 39.6...
-
A 7.53 L sample of N 2 (g) at 742 mmHg and 45.0 C is bubbled through CCl 4 (l) at 45.0 C. Assuming the gas becomes saturated with CCl 4 (g) what is the volume of the resulting gaseous mixture if the...
-
Wilson engages Ruth to sell Wilsons antique walnut chest to Harold for $2,500. The next day, Ruth learns that Sandy is willing to pay $3,000 for Wilsons chest. Ruth nevertheless sells the chest to...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Porch Pirates An InsuranceQuotes.com survey showed that 8% of Americans had a holiday package stolen from outside their front door. Consider the random selection of four Americans. Use the...
-
GATE 2024-EE Question
-
GATE 2024-EE Question
-
GATE 2024-EE Question
-
Place the following compounds (a) in order of increasing basicity and (b) in order of increasing acidity. NHCCH3 NH2 NH2 acetanilide cyclohexylamine aniline
-
CdF2 (s) Cd+ (aq) + 2 F- (aq) 1. A saturated solution of CdF2 is prepared. The equilibrium in the solution is represented above. In the solution [Cd+] eq = 0.0585 M and [F-] eq = 0.117 M. a....
-
Dividend Growth Model Based on the dividend growth model, what are the two components of the total return on a share of stock? Which do you think is typically larger?
-
Growth Rate In the context of the dividend growth model, is it true that the growth rate in dividends and the growth rate in the price of the stock are identical?
-
Voting Rights When it comes to voting in elections, what are the differences between US political democracy and U.S. corporate democracy?
-
CROSS RATES Suppose the exchange rate between the U.S. dollar and the Swedish krona was 6 krona = $1, and the exchange rate between the dollar and the British pound was 1 = $1.85. What would be the...
-
0 Suppose that two different studies (A and B) have the same sample sizes in e four groups, with similar standard deviations in the four groups. Furthermore sample sizes and sample SDs are also the...
-
Please answer both, its the same question but a 2 part answer. Southwest Milling Company purchased a front-end loader to move stacks of lumber. The loader had a list price of $121,930. The seller...

Study smarter with the SolutionInn App


