(A) Acetonitrile is an industrial solvent. Propose a hybridization and bonding scheme consistent with its structure. (B)...
Question:
(A) Acetonitrile is an industrial solvent. Propose a hybridization and bonding scheme consistent with its structure.
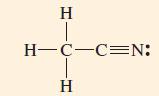
(B) A reference source on molecular structures lists the following data for dinitrogen monoxide (nitrous oxide), N2O: Bond lengths: N—N = 113 pm; N—O = 119 pm; bond angle = 180°. Show that the Lewis structure of N2O is a resonance hybrid of two contributing structures, and describe a plausible hybridization and bonding scheme for each.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





