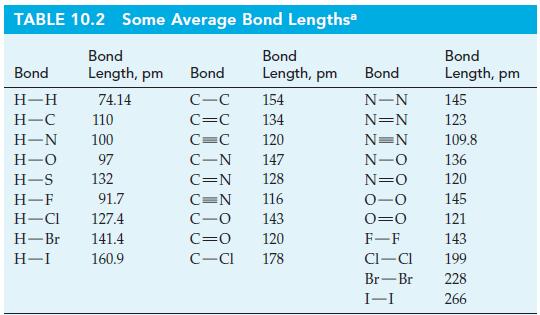
A relationship between bond lengths and single bond covalent radii of atoms. Use this relationship together with
Question:
A relationship between bond lengths and single bond covalent radii of atoms. Use this relationship together with appropriate data from Table 10.2 to estimate these single-bond lengths.
(a) I—Cl;
(b) O—Cl;
(c) C—F;
(d) C—Br.
Table 10.2
Transcribed Image Text:
TABLE 10.2 Some Average Bond Lengths Bond Bond Length, pm Length, pm Bond H-H H-C 110 H-N 100 H-O 97 H-S 132 H-F 91.7 H-Cl 127.4 H-Br 141.4 H-I 160.9 74.14 Bond C-C C=C C=C C-N C=N 154 134 120 147 128 116 143 120 C=N C-O C=0 C-Cl 178 Bond N-N N=N N=N N-O N=O 0-0 0=0 F-F CI-CI Br-Br I-I Bond Length, pm 145 123 109.8 136 120 145 121 143 199 228 266
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
The bond lengths of these bonds can be determined using the average bond lengths of the co...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use Table 8.4 to estimate the enthalpy change for each of the following reactions: a. H2C == O (g) + HCl (g) H3C - O - Cl (g) b. H2O2 (g) + 2CO (g) H2 (g) + CO2 (g) (c). 3H2C == CH2 (g) C6H12 (g)...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
A utilization greater than one suggests that the mean service time is higher than the mean inter-arrival time. True False QUESTION 3 It costs five times more money to retain a current customer than...
-
Ronald D. Johnson is a former employee of International Business Machines Corporation (IBM). As part of a downsizing effort, IBM discharged Johnson. In exchange for an enhanced severance package,...
-
Lees accountant recorded the depreciation on Lees cottage during 2011 as $7,000. What did the accountant say Lees profit or loss was? Lee, a programmer, earned $35,000 in 2010, but in 2011, he began...
-
We use the evolutionary approach as a metatheoretical framework to integrate the various perspectives on organizations. Are there some perspectives that fit more comfortably within an evolutionary...
-
Journalize the following entries for (1) the buyer and (2) the seller. Record all entries for the buyer first. 201X June 11 LePorte Company sold $9,000 of merchandise on account to Ramsey Company....
-
For each of the following questions choose a corresponding category of financial ratio that is commonly used. Asset management efficiency ratio Capital structure ratio Liquidity ratio Market value...
-
Cutler Corporation has a target capital structure of 55 % common stock, 5 % preferred stock, and 40 % debt. Its cost of equity is 16 %, the cost of preferred stock is 10 %, and the cost of debt is 9...
-
Estimate the lengths of the following bonds and indicate whether your estimate is likely to be too high or too low: (a) ICl; (b) CF.
-
Without referring to tables in the text, indicate which of the following bonds you would expect to have the greatest bond length, and give your reasons. (a) O 2 ; (b) N 2 ; (c) Br 2 ; (d) BrCl.
-
Why is a firms credit policy important for translating the revenue budget into the budgeted inflows of cash?
-
Using a ruler, completing the following schematics based on the information provided. 19. Draw a wiring diagram of a PSC compressor with a current start relay and a start capacitor.com 20. Draw a...
-
You observe the below command output. *?What is wrong connection timed out; no servers could be reached arya@arya:~$
-
Evaluate your strengths a supervisor and leader using the preferred leadership profile, the key performance motivators scale, the seven domains for inspiration, and other concepts from your course...
-
3) A spider crawls with constant speed vo on a phonograph turntable rotating with constant angular speed w in the xy plane on a radially outward path, relative to the centre of the turntable. The...
-
Question Encik Zubir ( a certified handicapped person ) is the owner of a financial consulting firm, Bijak Wealth Enterprise. The business assists its clients to grow their wealth. Encik Zubir is...
-
Which of the following bromides will react faster with methanol (via an SN1 reaction)? What are the reaction products in each case? a. b. CH3CH2CH2Br or H2C=CHCH2Br Br or Br
-
A genetically engineered strain of Escherichia coli (E. coli) is used to synthesize human insulin for people suffering from type I diabetes mellitus. In the following simplified reaction scheme,...
-
A factory costs $400,000. You reckon that it will produce an inflow after operating costs of $100,000 in year 1, $200,000 in year 2, and $300,000 in year 3. The opportunity cost of capital is 12...
-
Halcyon Lines is considering the purchase of a new bulk carrier for $8 million. The forecasted revenues are $5 million a year and operating costs are $4 million. A major refit costing $2 million will...
-
As winner of a breakfast cereal competition, you can choose one of the following prizes: a. $100,000 now. b. $180,000 at the end of five years. c. $11,400 a year forever. d. $19,000 for each of 10...
-
This is the solved part A. Just need help in solving the next questions from 1-6. These four pages are interconnected one by one as i posted. First part was solved as I uploaded the picture...
-
WHEN INVESTORS PREFER SHORT TERM INVESTMENTS THIS IS CALLED
-
You have been advised that the cost of ordinary equity is 8%, preference shares are 10% and pre-tax cost of debt is 7%. The weights of preference shares is 25% and ordinary shares are 45%. The tax...

Study smarter with the SolutionInn App


